Since January 2003, vaccination with Meningococcal C conjugate vaccine (MenCCV) is recommended at 12 months of age, at the same time as the measles-mumps-rubella (MMR) and Haemophilus influenzae type b (Hib) vaccines. Most (83%) of a cohort of 751 children in north Queensland born in January 2003 received the three injectable vaccines simultaneously. Of the 122 children who had not received MenCCV with the other two vaccines, 88 (72%) had received it by 18 months of age. The median age of receipt of MenCCV in the children who had received the three vaccines simultaneously was 12.3 months, whereas the median age in the children who had not received it at the same time as the other two vaccines was 14.0 months. This study suggests that non-simultaneous vaccination puts children at-risk of receiving MenCCV late, or not at all, and has implications for the introduction of universal infant pneumococcal vaccination program, starting in January 2005. Commun Dis Intell 2004;28:493–496.
Introduction
The meningococcal C conjugate vaccine (MenCCV) was introduced into the Australian Standard Vaccination Schedule (ASVS) in January 2003.1 The vaccine is recommended to be administered at 12 months of age, at the same time as the administration of the first dose of measles-mumps-rubella (MMR) and the third dose of Haemophilus influenzae type b (Hib) vaccine.
Since mid-2001, with the introduction of the seven-valent pneumococcal conjugate vaccine (7vPCV) it has been recommended that Indigenous children receive three vaccine injections at two and four months of age.2 However, the introduction of MenCCV is the first time that three simultaneous vaccine injections are recommended for all Australian children.
Little is known about the acceptability of three simultaneous injectable vaccines to Australian vaccine providers and parents of young children.3 If three simultaneous injections are considered ‘unacceptable’ they might be ‘split’, with two vaccines given at a first visit and the third at a second visit some time later. If this occurs, it is likely that the most recently introduced vaccine (in this case MenCCV) would be the vaccine given later and there is the possibility that the return visit might either occur late or not occur at all.
The aims of this study were to determine the percentage of a cohort of children in north Queensland that had received the three vaccines simultaneously, and to describe some of the characteristics of the children that did not receive the three vaccines simultaneously.
Top of page
Methods
The vaccination records of all children born in January 2003, who were resident in north Queensland when they were eligible for the three vaccines (i.e. from 12 months of age onwards), were extracted from the state-wide immunisation register (VIVAS). For each child the age when the three vaccines were administered, and whether they were given simultaneously, was recorded, as was the child’s Indigenous status.
Six vaccines—including three doses of Japanese encephalitis (JE) vaccine—are recommended on three visits in the 12th month of life for children on the outer islands of the Torres Strait. MenCCV is recommended for these children with the second dose of JE vaccine (one week after MMR and the first dose of JE vaccine). Therefore any outer island child who either had MenCCV simultaneously with the MMR and the first dose of JE vaccine, or (as recommended) simultaneously with the second dose of JE vaccine, was defined for the purpose of this study as having had the three vaccines simultaneously.
In cases where the Indigenous status of a child was not recorded, it was assumed that the child was Indigenous if he/she had received both BCG at birth and 7vPCV. The last known vaccine provider was contacted for any further clarification of a child’s Indigenous status.
If one or more of the three vaccines was not recorded as having been given to any child, the relevant vaccine provider was contacted and asked for further details. If the vaccine(s) had not been given, the provider was requested to recall the child for vaccination, and if that occurred to ensure that the relevant details were forwarded for entry onto VIVAS. If none of the three vaccines had been given, the relevant Public Health Nursing Officer was informed and requested to attempt to locate the child and, if successful, to request a vaccine provider to vaccinate the child.
The vaccination record extraction for the study commenced in March 2004, and was concluded at the end of July. By this time all the study children would have reached 18 months of age.
Top of page
Results
The study cohort consisted of 751 children, 165 (22%) of whom were Indigenous children. Seventeen of the Indigenous children were from the outer Torres Strait islands, only eight of whom had received the three injectable vaccines simultaneously (as defined in Methods above).
Four children, all of whom were Indigenous, had not received any of the three injectable vaccines by 18 months of age. Two children (both Indigenous) who were very overdue for various infant vaccines had had four simultaneous injectable vaccines including MMR and Hib but not MenCCV, as part of catch-up schedules, and the parent of another child had refused MMR (but not MenCCV or Hib) for her child. These latter three children therefore could not have had the three recommended vaccines—MMR, Hib and MenCCV—administered simultaneously.
Five children had received privately-purchased MenCCV in age-appropriate schedules in the first year of life. It was assumed that these children would otherwise have had MenCCV simultaneously with the MMR and Hib vaccines, which had indeed been given at the same time. Another seven children had been given a third dose of Hib vaccine at about six months of age, but all had received a fourth dose simultaneously with MMR and MenCCV.
Altogether, 99 per cent of the 751 children had received MMR, 98.5 per cent Hib, and 94.5 per cent MenCCV by 18 months of age. Very similar percentages of Indigenous (93%) and non-Indigenous children (95%) had received MenCCV by 18 months of age.
Altogether, 622 (83%) of the children had received the three injectable vaccines simultaneously. Virtually the same percentage of Indigenous (82%) and non-Indigenous (83%) children received the vaccines simultaneously.
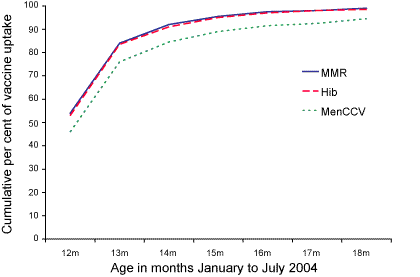
Of the 122 children who had not received MenCCV simultaneously with the other two vaccines, 88 (72%) had received it by 18 months of age. The median age of receipt of MenCCV in the children who had received the three vaccines simultaneously was 12.3 months, whereas the median age of receipt of MenCCV in the children who had not received it at the same time as the other two vaccines was 14.0 months. The cumulative uptake of the three vaccines by age is shown in the Figure.
The vaccine providers who gave MMR to the 122 children who did not receive the three vaccines simultaneously were general practitioners (90; 74%), Queensland Health community health staff (29; 24%) and other providers (3; 2%).
Figure. The cumulative percentage uptake of measles-mumps-rubella, Haemophilus influenzae type b and Meningococcal C conjugate vaccines in the study cohort, North Queensland, by month January to July 2004
Top of page
Discussion
A survey of parental and general practitioner attitudes to multiple vaccine injections in New South Wales in 1997 found that ‘only 54 per cent of parents and 28 per cent of GPs said they would allow three injections to be given at one visit’.3 However, this current study has demonstrated not only that most (83%) children received the three vaccines simultaneously, but also that the multiple vaccine recommendation (introduced in January 2003) had been rapidly accepted and implemented. This difference between the hypothetical attitudes and the practical realities is very similar to that seen in the United States of America prior to, and after, the introduction of inactivated polio vaccine (IPV) not combined with any other antigens.4–6
There are two possible reasons that might explain this difference between attitude and practice. Firstly, two (MMR and Hib) of the three vaccines were already relatively well known to parents and vaccine providers, and the other (MenCCV) was for the prevention of a disease that has attracted considerable adverse publicity in Australia in recent years. Secondly, attitudinal surveys indicated that many parents were likely to accept a recommendation of multiple simultaneous injections for their children from a vaccine provider,3,4 and clearly most providers are comfortable not only with recommending multiple vaccine injections but also administering them.
However, it is of considerable concern that only 72 per cent of the 122 children who did not receive the three vaccines simultaneously had received the MenCCV by 18 months of age. Indeed, this figure is likely to be higher than expected for two reasons: a letter from the Australian Government’s Chief Health Officer was sent in June 2004 to all parents of young children who had (apparently) not received MenCCV informing them of the availability of this vaccine free for their children, and the study process itself involved directly contacting vaccine providers and informing them to recall children who had not yet received the vaccine. Without these two reminders, it is likely that even fewer children would have received MenCCV by 18 months of age.
This study has demonstrated that non-simultaneous vaccination puts children at-risk of receiving MenCCV late, or not at all. It also confirms that it is the most recently recommended vaccine, in this case MenCCV, is the most likely to be administered separately from the other vaccines, so as to avoid more than two injections at the same time.
The findings of this study have implications for the introduction of universal infant 7vPCV, to commence in January 2005. This change to the ASVS will mean that three vaccine injections (in Queensland: DTPa-hepB, Hib, 7vPCV) are recommended for all children at two and four months of age. The exception would be if an infant’s parents were prepared to purchase the hexavalent DTPa-hepB-IPV-Hib vaccine, to be administered simultaneously with 7vPCV at two, four and six months of age. This option is not considered suitable for Indigenous children.
Severe invasive pneumococcal disease can occur early in life, even in apparently low-risk infants.7 For example, in 21 cases of pneumococcal meningitis in low-risk non-Indigenous infants in north Queensland, the median age of these children was 11 (range 4–24) months of age. Two children developed the meningitis before six months of age, and 10 (48%) had onsets before eight months of age. Therefore, it is imperative that there not be any unnecessary delays in administering 7vPCV if the vaccine is to have an optimal impact in preventing invasive pneumococcal disease.
The recommendations to administer MenCCV simultaneously at 12 months of age, and 7vPCV simultaneously at two and four months of age were made after consideration of evidence concerning adverse events and immune responses following the simultaneous administration of the relevant vaccines.8 Vaccine providers, in particular general practitioners, need to be reassured that the available evidence indicates that these vaccines, when given simultaneously as recommended, are safe and effective. Quite simply, the most important factor influencing parents to agree to multiple injectable vaccines for their children is a confident and positive recommendation from the vaccine provider.
Top of page
Acknowledgements
We wish to thank Brad McCulloch, Kathy Lort-Phillips and Brigitte Dostie for their assistance with this study. We also wish to thank all the vaccine service providers who provided us with information relevant to the study.
Top of page
References
1. Cohen NJ. Introduction of the National Meningococcal C Vaccination program (editorial). Commun Dis Intell 2003;27:161–162.
2. Hanna JN, Bullen RC, Ziegler CL, et al. An assessment of the implementation of the pneumococcal conjugate vaccination program for Aboriginal and Torres Strait infants in North Queensland. Commun Dis Intell 2002;26:262–266.
3. Bartlett MJ, Burgess MA, McIntyre PB, Heath TC. Parent and general practitioner preferences for infant immunisation. Reactogenicity or multiple injections? Aust Fam Physician 1999;28(Suppl 1):S22–S27.
4. Woodin KA, Rodewald LE, Humiston SG, Carges MS, Schaffer SJ, Szilagyi PG. Physician and parent opinions. Are children becoming pincushions from immunizations? Arch Pediatr Adolesc Med 1995;149:845–849.
5. Melman ST, Nguyen TT, Ehrlich E, Schorr M, Anbar RD. Parental compliance with multiple immunization injections. Arch Pediatr Adolesc Med 1999;153:1289–1291.
6. Kolasa MS, Petersen TJ, Brink EW, Bulim ID, Stevenson JM, Rodewald LE. Impact of multiple injections on immunization rates among vulnerable children. Am J Prev Med 2001;21:261–266.
7. Fagan RL, Hanna JN, Messer RD, Brookes DL, Murphy DM. The epidemiology of invasive pneumococcal disease in children in Far North Queensland. J Paediatr Child Health 2001;37:571–575.
8. National Health and Medical Research Council. The Australian Immunisation Handbook. 8th ed. Canberra: Commonwealth of Australia, 2003.
Top of page
Author affiliations
1. Public Health Physician, Tropical Public Health Unit, Cairns, Queensland
2. Public Health Nursing Officer (Immunisation), Tropical Public Health Unit Network, Queensland Health, Cairns, Queensland
3. Information Systems Coordinator, Tropical Public Health Unit Network, Queensland Health, Cairns Queensland
Corresponding author: Dr Jeffrey Hanna, Tropical Public Health Unit, PO Box 1103, Cairns Queensland 4870. Telephone: +61 7 4050 3604. Facsimile: +61 7 4031 1440. Email: Jeffrey_hanna@health.qld.gov.au
This article was published in Communicable Diseases Intelligence Vol 28 No 4, December 2004.
