The Australian Mycobacterium Reference Laboratory Network collected and
analysed laboratory data on new cases of disease caused by Mycobacterium tuberculosis complex in the year
2003. A total of 784 cases were identified by bacteriology, representing
an annual reporting rate of 3.9 cases of laboratory confirmed tuberculosis
per 100,000 population. The most commonly encountered
culture-positive specimens were sputum (n=351), lymph node (n=176) and from
bronchoscopy (n=97). Smears containing acid fast bacilli were present in
sputum (53.0%), bronchoscopy (32.0%) and lymph node (23.3%). Five children
(female n=3, male n=2) under 10 years of age had bacteriologically confirmed
tuberculosis. Eighty isolates of M.
tuberculosis and one of Mycobacterium africanum
(10.3%) were resistant to at least one of the standard anti-tuberculosis
agents. Mono-resistance to isoniazid, ethambutol, rifampicin, and pyrazinamide
was detected in 45, three, two, and one isolates respectively. Multidrug-resistance
(MDRTB) defined as resistance to both isoniazid and rifampcin was observed
in seven (0.9%) isolates. Of the seven MDRTB isolates, six were from the
respiratory tract and four were from smear positive specimens. Of the 81
patients with drug resistant isolates, 78 (96.3%) were classified as having
initial resistance; two had acquired resistance and no information was available
for one isolate; five were Australian-born; and 76 (93.8%) had migrated from
a total of 30 countries. Commun Dis Intell 2004;28:474–480.
Top of page
Introduction
The annual incidence of tuberculosis (TB) diagnosed clinically in Australia has fallen
from 55 cases per 100,000 population in the mid 1950s to a current level
around 5 to 6 cases per 100,000 population. As part of the Western Pacific
region of the World Health Organization, Australia enjoys one of the lowest rates of disease
compared with the rest of the region which reported an overall notification
rate of 47 per 100,000 population in year 2002.
This rate has shown no significant variation since 1993.3
The Western Pacific region contains several countries (China, Philippines, Viet
Nam, Cambodia and Papua New Guinea) with a high burden of TB. Another
regional neighbor, the Republic of Indonesia, has the third highest
burden of TB in the world.2
There are two sources of TB-related data for Australia. Since 1991, the National Notifiable
Diseases Surveillance System (NNDSS) has provided statistics on cases of
tuberculosis reported to public health authorities in Australia’s states and territories.
The second source, the Australian Tuberculosis Reporting Scheme has been
conducted by the Australian Mycobacterium Reference Laboratory Network (AMRLN)
since 1986. Statistics compiled by the AMRLN relate to cases of bacteriologically
confirmed tuberculosis whereas NNDSS data will have a proportion of cases
that are identified on the basis of clinical and epidemiological information,
or on non-bacteriological laboratory investigations. This report describes
the bacteriologically confirmed TB diagnoses for the year 2003.
Top of page
Methods
The data are based on clinical specimens that were culture-positive for
Mycobacterium tuberculosis
complex (MTBC). Although the BCG strain of Mycobacterium bovis
is a member of the MTBC, no information on this organism is included in the
present report. Almost all isolates of MTBC were referred to one of the five
laboratories comprising the AMRLN for specific identification and drug susceptibility
testing. Comparable methodologies are used in the reference laboratories.
Relapse cases, as defined by the National Strategic
Plan for TB Control in Australia beyond 2000 prepared by the
National TB Advisory Committee, were included in the laboratory data as laboratories
are generally unable to differentiate relapse cases from new cases.3
Temporary visitors to Australia were included as were illegal aliens within
correctional services facilities and asylum seekers located in detention
centres or on temporary visas within Australia.
For each new bacteriologically confirmed case, the following information
was collected (where available):
- demography: patient identifier, age, sex, HIV status and state of residence;
- specimen: type, site of collection, date of collection and microscopy result;
- isolate: species of mycobacterium and results of drug susceptibility testing;
- nucleic acid amplification testing: results of testing; and
- if the isolate was drug resistant: patient country of origin, and history of previous TB treatment to determine whether resistance was initial or acquired.
Data from contributing laboratories were submitted in standard format
to the scheme coordinator for collation and analysis. Duplicate entries (indicated
by identical patient identifier and date of birth) were deleted prior to
analysis. Rates were calculated using mid-year estimates of the population
for the year 2003 supplied by the Australian Bureau of Statistics.4
For each case, the nature of the first clinical specimen that yielded
an isolate of MTBC was used to record the nominal site of disease. Culture-positive
specimens collected at bronchoscopy or by gastric lavage were considered
to indicate pulmonary disease. Cases with multi-site isolations, provided a sputum or bronchoscopy specimen was
culture-positive, were listed as having pulmonary disease, the most important
category for public health purposes. Cases for which there were multiple-site
isolations were not categorised as having miliary or disseminated disease
as differentiation is based on clinical findings that are generally not available
to the reporting laboratories. Initial drug resistance was defined as the
presence of drug resistant strains of M.
tuberculosis and M.africanum in cases
of tuberculosis in which there was no known history of anti-tuberculosis
treatment. Patients who had begun anti-TB treatment and had developed resistance
to one or more of the drugs used during treatment were recorded as having
acquired drug resistance.5
Top of page
Results
There were 784 bacteriologically confirmed cases of tuberculosis in 2003
(Figure 1), representing an annual rate of 3.9 per 100,000 population. State-specific reporting rates varied from 0.8 cases (Tasmania) to 10.1 cases per 100,000 population (Northern Territory) (Table 1).
Figure 1. Comparison between tuberculosis notifications and laboratory data, Australia; 1990 to 2003
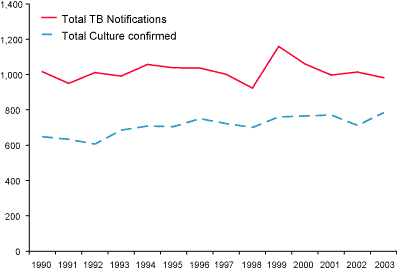
Table 1. Bacteriologically confirmed cases of tuberculosis in Australia, 1994 and 2000 to 2003, cases and rate per 100,000 population by state or territory*
| State or territory |
2003 |
200216 |
200115 |
200014 |
199410 |
| n |
Rate |
n |
Rate |
n |
Rate |
n |
Rate |
n |
Rate |
| New South Wales † |
325 |
4.6 |
301 |
4.3 |
327 |
4.8 |
307 |
4.5 |
278 |
4.4 |
| Victoria |
254 |
5.2 |
208 |
4.3 |
222 |
4.6 |
231 |
4.8 |
217 |
4.8 |
| Queensland |
91 |
2.4 |
97 |
2.6 |
81 |
2.2 |
76 |
2.1 |
88 |
2.8 |
| Western Australia |
54 |
2.8 |
46 |
2.4 |
68 |
3.6 |
63 |
3.3 |
53 |
3.1 |
| South Australia |
36 |
2.4 |
26 |
1.7 |
38 |
2.5 |
41 |
2.7 |
41 |
2.8 |
| Tasmania |
4 |
0.8 |
8 |
1.7 |
12 |
2.8 |
2 |
0.4 |
10 |
2.1 |
| Northern Territory |
20 |
10.1 |
26 |
13.0 |
23 |
11.6 |
45 |
23.0 |
21 |
12.3 |
| Total |
784 |
3.9 |
712 |
3.6 |
771 |
4.0 |
765 |
4.0 |
708 |
4.0 |
Top of page
Causative organism
Almost all isolates were identified as M.
tuberculosis (n=782), the remaining two isolates being a single
M. africanum and a M. bovis.
Distribution by gender, age and site of disease
Complete information for gender and age were submitted for all patients,
due to additional information provided by state and territory Tuberculosis
Centres. Five children (female n=3 male n=2) under 10 years of age had bacteriologically
confirmed tuberculosis (lymph node n=2, tracheal aspirate n=1, gastric aspirate
n=1, biopsy n=1).
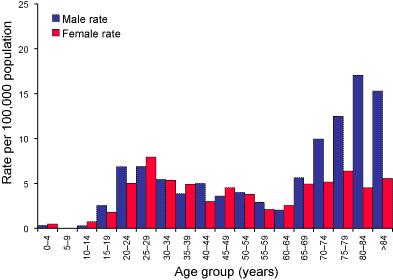
The relationship of tuberculosis to age and gender are shown in Figure
2. For males, there were two distinct age groups; a rise to 6.9 cases of
tuberculosis per 100,000 population at 20–24 and 25–29 years, and in the
elderly male where the rate rose from 5.6 at age grouping 65–69 to a peak
of 17.1 per 100,000 population for the 80–84 age group. Females in the 25–29
year age group had a peak rate of 8.0 per 100,000 population but
in contrast to males, the rate for tuberculosis in the elderly female was
more modest rising only to 6.4 cases per 100,000 population. In part, these
differences are due to the site of infection. Overall, the male:female ratio was 1.16:1, for sputum isolates, but the ratio
was reversed for lymph node isolates (1:1.4). The median age group for patients
with respiratory disease was 35–39 for females and 45–49 for males, and for
lymph node cases, the median age group for both genders was 35–39 years.
The predominant specimen type was sputum, including three gastric aspirates
(n=351, 44.7%); bronchoscopy (n=97, 12.4%), lymph node (n=176, 22.4%) and
pleural (n=35, 4.5%) (Table 2).
Table 2. Site of specimens smear- and culture-positive for Mycobacterium tuberculosis complex, in the year 2003
| |
n* |
Smear positive (%)† |
| Sputum |
351 |
186 (53.0) |
| Bronchoscopy |
97 |
31 (32.0) |
| Lymph node |
176 |
41 (23.3) |
| Pleural |
35 |
2 (5.7) |
| Genito-urinary |
18 |
9 (50.0) |
| Bone/Joint |
25 |
9 (36.0) |
| Peritoneal |
24 |
2 (8.3) |
| Skin |
11 |
ND † |
| CSF |
6 |
ND † |
† Percentage of specimens smear positive not calculated due to small numbers.
Association with HIV
The AMRLN database recorded the HIV status for only 55 (7.0%) patients.
Only two patients were identified as HIV seropositive; one had smear-positive
respiratory disease and the other patient had genitourinary TB.
Top of page
Microscopy
Results of microscopy were available for 751 of 784 (95.8%) of specimens;
microscopy was not performed on seven specimens and no results were provided
for the remaining 26 specimens. Smears were positive for 186 of 351 (53.0%)
sputum and 31 of 97 (32.0%) bronchoscopy specimens respectively (Table 2).
A total of 35 pleural specimens (8 biopsy and 27 fluids) were culture positive
for M. tuberculosis, but
only one of each specimen type was smear positive. Lymph node specimens were smear positive
for only 41 of 176 (23.3%) cases.
Figure 2. Laboratory confirmation of Mycobacterium tuberculosis complex disease, Australia 2003, by age and sex.
Drug susceptibility testing
Results of in vitro
drug susceptibility testing were available for all 784 isolates for isoniazid
(H), rifampicin (R) and ethambutol (E) and for 783 isolates for pyrazinamide
(Z). A total of 81 isolates (10.3%) of M.
tuberculosis (n=80) and M. africanum
(n=1) were resistant to at least one of the above anti-tuberculosis agents.
Results of testing for streptomycin (S) were available for 222 of 784 (28.3%)
of isolates with nine demonstrating S mono-resistance and another eight were
resistant to S + H. Resistance to at least both H and R (defined as multidrug
resistance) was detected in seven (0.9%). All of the MDR isolates were M. tuberculosis (Table 3). Of the 7 MDRTB isolates,
six were from the respiratory tract (sputum n=4, bronchoscopy n=2); the remaining
isolate was from a lymph node. Three of the MDRTB-positive sputum specimens
were smear positive as was one of the bronchoscopy specimens and the single
isolate from lymph node tissue. A single isolate of M. bovis from a smear-positive sputum was not
included in the above results.
Table 3. Drug resistance patterns in MDR strains, Australia, 1993 to 2003
Resistance pattern (standard drugs)* |
2003 |
200216 |
200115 |
200014 |
199913 |
199813 |
199712 |
199611 |
199510 |
199410 |
19939 |
| H+R‡ only |
4 |
8 |
8 |
3 |
2 |
2 |
6 |
10 |
3 |
2 |
7 |
| H+R+E‡ |
2 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
0 |
|
| H+R+Z‡ |
1 |
1 |
3 |
3 |
1 |
2 |
5 |
4 |
1 |
0 |
|
| H+R+E+Z‡ |
0 |
2 |
0 |
1 |
0 |
1 |
0 |
0 |
0 |
0 |
1 |
Total (%) |
7
(0.9) |
12
(1.7) |
12
(1.6) |
8
(1.0) |
4
(0.5) |
6
(0.9) |
14
(1.9) |
15
(2.0) |
5
(0.7) |
2
(0.3) |
10†
(1.5) |
Mono-resistance to isoniazid, ethambutol, rifampicin, and pyrazinamide
was detected in 45, three, two, and one isolates respectively. There were
75 isolates that demonstrated resistance to H at a concentration of 0.1 mg/L.
Of these, 41 (54.7%) demonstrated resistance to H at the higher level of
0.4 mg/L. Thirty-seven of 81 (45.7%) specimens culture-positive for drug
resistant M. tuberculosis, including 26 of 55 (47.3%)
sputum or bronchoscopy specimens, were smear-positive for AFB. Six of the
seven MDRTB isolates had high level isoniazid resistance.
Top of page
Initial or acquired resistance, and country of origin
There were 80 M. tuberculosis
and one M. africanum resistant to at least one of
the standard drugs (H, R, E, Z). Of these, 78 of 81 (96.3%) were classified
as having initial resistance, two had acquired resistance, and no data was
available for one isolate on the presence or absence of previous treatment.
The country of birth was known for all patients with drug resistant strains;
five were Australian born, and 76 (93.8%) had migrated from a total of 30
countries.
Of the 76 migrants with drug-resistant disease, 49 (64.5%) had migrated
from one of six countries; Viet Nam (n=18), India (n=8), Philippines (n=7), Indonesia (n=5), Sudan (n=5),
and China (n=4).
Use of nucleic acid amplification tests
Nucleic acid amplification testing (NAAT) was performed on 201 of 784
(25.6%) specimens, all of which subsequently grew M.
tuberculosis on culture. Of these, 123 specimens were of respiratory
origin (sputum, n=90, bronchoscopy, n=26, tissue, n=4, aspirate, n=3), and
112 (91.1%) were NAAT positive. For smear positive respiratory specimens,
80 of 83 (96.4%) were NAAT positive whilst 26 of 32 (81.3%) of smear negative
respiratory specimens were NAAT positive (Table 4A). Seven specimens did
not record a smear result and one smear negative tissue specimen recorded
an equivocal result.
Table 4A. Results for nucleic acid amplification tests performed on respiratory specimens, Australia,
2003
| NAAT result |
Culture positive respiratory specimens |
| Smear positive |
Smear negative |
| Positive |
80 |
26 |
| Negative |
3 |
6 |
| Total* (115) |
83 |
32 |
There were 78 specimens of non-respiratory origin (tissue, n=50, aspirate,
n=14, fluid, n=13, swab, n=1) and only 47.4 per cent were NAAT positive.
For smear positive non-respiratory specimens, 19 of 22 (86.4%) were NAAT
positive and 18 of 51 (35.3%) of smear negative non-respiratory specimens
were NAAT positive (Table 4B). Four specimens did not record a smear result
and one smear-positive spinal tissue specimen recorded the presence of NAAT
inhibitors.
Top of page
Table 4B. Results for nucleic acid amplification tests performed on non-respiratory specimens, Australia,
2003
|
AAT result |
Culture positive NON-respiratory specimens |
| Smear positive |
Smear negative |
| Positive |
19 |
18 |
| Negative |
3 |
33 |
Total* (73) |
22 |
51 |
Top of page
Discussion
The finding of 784 cases of bacteriologically confirmed tuberculosis
representing 3.9 cases per 100,000 population in
2003 is consistent with the results of previous AMRLN reports. Since the
network began collecting data in 1986, the range for bacteriologically confirmed
cases has remained between 3.5–4.1 per 100,000 population.6–16
For 2003, the NNDSS reported 982 notified cases of TB, a difference between
the two datasets of 198 (25.3%).17 The NNDSS has
consistently recorded a higher number of notifications than the AMRLN data
(range 22.7–44%). Possible reasons for the gap between the two data sources
have been discussed previously.14
Furthermore, the handling of multiple sites of disease differs also. The
NNDSS database documents all sites of disease, whereas the AMRLN database
lists only one site, and when multi-site disease is present, prioritises
respiratory disease over non-respiratory sites. Although comparison of the
unlinked databases is problematic, there were 483 and 236 notifications of
respiratory and lymph node disease respectively in 2003.17
The AMRLN dataset recorded 351 respiratory and 176 lymph node cases. If the two datasets are compared, then 74.7 per cent and 74.6 per
cent of respiratory and lymph node notifications respectively were bacteriologically
confirmed. Over the period, 2000–2003, the range of bacteriologically
confirmed respiratory or lymph node disease was 70.5–88.5 per cent or 63.5–86
per cent respectively14–16, 18–20
In 2003, almost all isolates were identified as M. tuberculosis (n=782), the remaining two isolates
being a single M. africanum and an M.
bovis. In the past decade, the absolute number of cases caused
by M. bovis has fallen from a high of 10 and nine
cases in 1996 and 1997 respectively down to four, two, one, zero, and one
cases in the years 1999–2003. The number of cases caused by M.
africanum has remained at a steady, low level between zero and
seven cases per year over the past decade. Hence, a positive result by a
rapid method that detects the presence of MTBC in a clinical specimen most
likely indicates M. tuberculosis
rather than any other member of the MTBC.8–16
A total of 81 isolates (10.3%) of M.
tuberculosis (n=80) and M. africanum
(n=1) were resistant to one at least one of H, R, E, or Z. This finding is
consistent with previous reports provided by the AMRLN where drug resistance
has remained between a high of 17.7 per cent in 1989 and a low of 7 per cent
in 1994.6–16 For 2003, mono-resistance to isoniazid,
ethambutol, rifampicin, and pyrazinamide was detected in 45, three, two,
and one isolates respectively. Again, this finding is consistent with previous
data.
The level of acquired resistance in Australia remains low with only 2/81 (2.5%) cases
with a drug resistant strain being described as such. Interestingly, both
cases were MDRTB, one from Papua New Guinea and
the other from India. Most cases with drug resistant strains
(93.8%) occurred in the overseas born and reflects previous data.14–16
These findings reflect more upon the performance of the TB program from their
country of origin rather than the clinical management of these patients in
Australia. Therefore, as a measure of performance of Australia’s
TB control program, the national drug resistance data has limited usefulness.
Results of NAAT were evaluated with smear result and whether the sample
was from respiratory or non-respiratory sites. Consistent with previous reports,
96.4 per cent of smear- and culture- positive respiratory specimens were
NAAT-positive.21–23 Importantly, 3/83 (3.6%) of
smear positive respiratory specimens that subsequently grew MTBC were NAAT
negative and only 35.3 per cent of smear-negative culture positive non-respiratory
specimens were NAAT-positive. Inhibitors of amplification enzymes may be
present in any specimen, especially those of non-respiratory origin. Clinicians
must recognise the limited sensitivity of NAAT particularly on non-respiratory
samples and laboratorians must remember that NAAT should have an internal
amplification inhibitor control to validate a negative result.23,24
NAAT should be considered a supplemental test that does not replace microscopy
or culture. Culture also remains the priority because an MTBC isolate is
required for specific identification to species level, drug susceptibility
testing and genotyping.
Top of page
The decision to perform NAAT on a specimen needs to consider several
factors, including whether a sufficient amount of specimen has been set aside
for microscopy and culture, the degree of clinical suspicion for TB, and
the specimen type.21,24 Public health considerations
can also influence the decision to perform NAAT. For respiratory smear-positive
with no risk factors for TB, the differential diagnosis also includes disease
caused by environmental mycobacteria. A negative NAAT result in this setting
supports the diagnosis of NTM disease for which the drug treatment is different,
and the public health actions of isolation and contact tracing may be unnecessary.
Smear-negative patients may also be suitable candidates for NAAT when the
clinical suspicion of TB is moderate to high and multiple sputum specimens
are smear negative NAAT may clarify the diagnosis without resorting to further,
more-invasive investigations such as bronchoscopy. In contrast, smear negative
respiratory specimens from patients with a low probability of TB are not
suitable candidates for NAAT due to the test’s low sensitivity for the diagnosis
of smear negative pulmonary TB.21,22,23
For the first time, sufficient data was available to evaluate results
of NAAT on non-respiratory specimens. As expected, the correlation for smear
positive, non-respiratory specimens that were MTBC culture positive and NAAT
positive was lower at 86.4 per cent, most likely due to the presence of inhibitors.
For smear negative, non-respiratory specimens that were MTBC culture positive,
only 18/51 (35.3%) were NAAT positive. The level of sensitivity for NAAT
lies somewhere between that of culture (~10-100 colony forming units per
mL) and microscopy (~10,000 acid fast bacilli per mL) and the majority of
false-negative results are due to low concentrations of MTBC.25
Non-respiratory specimens generally have a far lower smear-positivity rate
than respiratory specimens (e.g. Table 2). Specimens from non-respiratory
sites such as tissue samples or fluids from usually sterile sites (e.g. cerebrospinal,
meningeal, pleural, ascitic, pericardial) tend to be paucibacilliary and
also have a higher proportion of specimens containing amplification inhibitors.
There are circumstances, most notably when meningeal TB is suspected, that
requests for NAAT are received. Only when sufficient specimen has been processed
for microscopy and culture should NAAT be considered.25,26
There is no place for using NAAT for checking the response to treatment.
NAAT does not differentiate nucleic acid from viable and non-viable MTBC
and furthermore, MTBC nucleic acid may remain in situ for an extended period of time. The
Centers for Disease Control and Prevention also recommended that NAAT should
not be used on specimens from patients who have received greater than seven
days of specific anti–TB treatment or have been on treatment within the previous
two months.24
In summary, the 2003 AMRLN database on positive TB cultures shows a steady
rate of laboratory-proven TB disease in Australia. The prevalence of drug-resistant disease
also remains unchanged. Most patients with drug-resistant TB were migrants
hence the rate of drug-resistant disease in Australia is an unreliable
performance indicator for our national TB control program. Finally, the AMRLN
database has provided further evidence on the performance characteristics
of NAAT. These findings confirm that NAAT should not be performed automatically
on every TB specimen or TB suspect. Furthermore, as with all mycobacterial
investigations, the decision to perform NAAT and the result interpretation
requires close liaison between the clinician and laboratory staff.
Top of page
Acknowledgements
The Australian Mycobacterium Reference Laboratory Network comprises the Mycobacterium Reference Laboratories at the following facilities:
- Institute of Medical and Veterinary Science, Adelaide, South Australia.
- Queensland Health Pathology Services, The Prince Charles Hospital, Chermside, Queensland.
- Victorian Infectious Diseases Reference Laboratory, North Melbourne, Victoria.
- Western Australian Centre for Pathology and Medical Research, The Queen Elizabeth II Medical Centre, Nedlands, Western Australia.
- Institute of Clinical Pathology and Medical Research, Westmead Hospital, Westmead, New South Wales.
Additional information and support from Ms Amanda Christensen, Dr Ral Antic, Dr Vicki Krause, Dr Graham Tallis, Dr Anastasios Konstantinos, and Dr Justin Waring, is gratefully acknowledged.
Top of page
References
1. World Health Organization.
Tuberculosis control in the WHO Western Pacific Region: 2004 report. ISBN
92 9061 090 5.
2. World Health Organization. Global
tuberculosis control WHO report 2001. WHO/CDS/TB
2001.275. Geneva, World Health Organization.
3. Communicable Diseases
Network Australia. National
Strategic Plan for TB Control in Australia Beyond 2000. Commonwealth
Department of Health and Ageing, Canberra, July 2002.
4. Australian Bureau
of Statistics. Australian Demographic Statistics, June Quarter 2003.
5. World Health Organization.
International Union Against TB and Lung Disease
Global Project on Anti-tuberculosis drug resistance. 2001 WHO/CDS/TB/2001. Geneva,
World Health Organization.
6. Dawson D, Anargyros
P, Blacklock Z, Chew W, Dagnia H, Gow B, et al. Tuberculosis in Australia:
an analysis of cases identified in reference laboratories in 1986–88. Pathology 1991;23:130–134.
7. Dawson D, Cheah DF, Chew F, Haverkort F, Lumb R, Sievers AS. Tuberculosis
in Australia,
1989–1992. Bacteriologically confirmed cases and drug resistance. Med
J Aust 1995;162:287–290.
8. Curran M, Dawson D,
Cheah D. Laboratory surveillance of Mycobacterium tuberculosis
isolates in Australia, 1992. Commun Dis Intell
1994;18:337–339.
9. Curran M, Dawson D.
Tuberculosis in Australia:
bacteriologically confirmed cases and drug resistance, 1993. Commun Dis Intell
1995;19:343–345.
10. Dawson D. Tuberculosis in Australia: bacteriologically
confirmed cases and drug resistance, 1994 and 1995. Commun Dis Intell
1997;21:245–249.
11. Dawson D. Tuberculosis in Australia; bacteriologically
confirmed cases and drug resistance, 1996. Commun Dis Intell
1998;22:183–188.
12. Dawson D. Tuberculosis in Australia; bacteriologically
confirmed cases and drug resistance, 1997. Commun Dis Intell
1999;23:349–353.
13. Dawson D. Tuberculosis in Australia: bacteriologically
confirmed cases and drug resistance, 1998 and 1999. Commun Dis Intell
2001;25:261–265.
14. Lumb R, Bastian I, Dawson D,
Gilpin C, Haverkort F, James G, Sievers A. Tuberculosis
in Australia:
bacteriologically confirmed cases and drug resistance, 2000. Commun Dis Intell
2002;26:226–233.
15. Lumb R, Bastian I, Dawson D,
Gilpin C, Haverkort F, James G, Sievers A. Tuberculosis
in Australia:
bacteriologically confirmed cases and drug resistance, 2001. Commun Dis Intell
2003;27:173–180.
16. Lumb R, Bastian I, Chew W, Gilpin
C, Haverkort F, Sievers A. Tuberculosis in Australia: bacteriologically
confirmed cases and drug resistance, 2002. Commun Dis Intell
2003;27:458–464.
17. Li J, Roche P, Spencer J, National TB Advisory Committee. Tuberculosis notifications in Australia, 2003. Commun Dis Intell 2004;28:464–474.
18. Lin M, Spencer J, Roche P, McKinnon M, National TB Advisory Committee. Tuberculosis notifications in Australia, 2000. Commun Dis Intell 2002;26:214–225.
19. Miller M, Lin M, Spencer J, National
TB Advisory Committee. Tuberculois notifications in Australia, 2001. Commun
Dis Intell 2002;26:525–536.
20. Samaan G, Roche P, Spencer J,
National TB Advisory Committee. Tuberculosis notifications
in Australia,
2002. Commun Dis Intell
2003;27:449–458.
21. Barnes PF. Rapid diagnostic tests
for tuberculosis: progress but no gold standard. Am
J Respir Care Med 1997; 155: 1497–1498.
22. Catanzaro A, Perry S, Clarridge JE, et al. The role of clinical suspicion in evaluating a new diagnostic test
for active tuberculosis. JAMA 2000; 283: 639–645.
23. Piersimoni C, Scarparo C. Relevance
of commercial amplification methods for direct detection of Mycobacterium tuberculosis complex in clinical
samples. J Clin Microbiol
2003; 41: 5355–5365.
24. Centers for Disease Control and
Prevention. Update: Nucleic acid amplification tests for tuberculosis. Morb Mortal Wkly Rep
2000; 49: 593–594.
25. Ausina V, Gamboa F, Gazapo E,
et al. Evaluation of the semi-automated Abbott
LCx Mycobacterium tuberculosis
assay for direct detection of Mycobacterium tuberculosis in
respiratory specimens. J Clin Microbiol 1996;35:1996–2002.
26. Moore DF, Curry JI. Detection and identification of Mycobacterium tuberculosis
directly from clinical sputum sediments by ligase chain reaction. J
Clin Microbiol 1998;36:1028–1031.
Top of page
Author affiliations
1. Infectious Diseases Laboratories, Institute of Medical and Veterinary Science, Adelaide, South Australia
2. Australian Mycobacterium Reference Laboratory Network
Corresponding author: Mr Richard Lumb, Principal Medical Scientist, Mycobacterium
Reference Laboratory, Infectious Diseases Laboratories, Institute of Medical and Veterinary Science, PO Box 14, Rundle Mall, Adelaide, South Australia 5000. Telephone: +61 8 8222 3579. Facsimile: +61 8 8222 3543. Email: richard.lumb@imvs.sa.gov.au
This article was published in Communicable Diseases Intelligence Vol 28 No 4, December 2004
