Megan K Young,1 Bradley J McCall,2 Helen V Smith,3 David Looke4
The Brisbane Southside Public Health Unit, received notification of a case of probable meningococcal septicaemia in a 22-year-old female on the 25th May 2004. Onset of illness was the 23rd May. Symptoms included lethargy, malaise and headache. On presentation, the patient was febrile and hypotensive, with an extensive purpuric rash. The patient responded to appropriate treatment and made a full recovery complicated by post infectious polyarthritis. The diagnosis was confirmed with positive blood cultures for Neisseria meningitidis. Five household contacts received prophylactic antibiotics on the 25th May, including the case’s 2-year-old child. Other (non-household) contacts were provided with information after confirmation of the diagnosis on the 26th May.
On the 27th May 2004, the public health unit received notification that Neisseria meningitidis had been isolated from an eye swab. The swab was taken on the 22nd May from the right eye of the case’s 2-year-old child. Investigation revealed that this child had developed purulent conjunctivitis on the 21st May after an upper respiratory illness of approximately one week’s duration.
The parent case had been interstate for the duration of the child’s conjunctival symptoms. The child had been taken by carers to the GP on the 22nd May in response to increasing respiratory symptoms. The GP prescribed Cefaclor (Ceclor®) for the child’s respiratory infection and chloramphenicol eye drops for the conjunctivitis. At the time of notification, the child was well and had completed a two day course of rifampicin in addition to the chloramphenicol eye drops. The course of Cefaclor (Ceclor®) had yet to be completed. The child was up to date with vaccinations including the conjugate meningococcal C vaccination that had been administered six months earlier.
Two additional close contacts were identified in relation to the child case. Both had received prophylaxis at the time of the parent case’s diagnosis, although this had not been initially recommended by public health. Two other social contacts were given information.
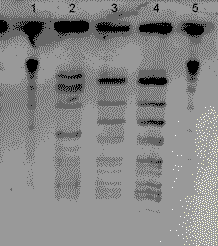
Isolates from the parent and child were confirmed as Neisseria meningitidis serogroup C 2a, p 1.5. The blood isolate from the parent case and the conjunctival isolate from the child were compared using pulsed field gel electrophoresis. They were found to be identical (Figure).
Figure. Pulsed field gel electrophoresis patterns of Neisseria meningitidis isolates from child and parent cases
Top of page
This family cluster highlights a number of points of public health importance. The first concerns the protection afforded by the conjugate meningococcal C vaccine. The efficacy of this vaccine has been estimated as 90 per cent and 96 per cent by two different groups in relation to invasive disease in the United Kingdom.1,2 Immunological memory after vaccination has been well documented using serum antibody responses.3,4 The efficacy of the vaccine with respect to generating mucosal antibody response is unknown. Zhang et al found significant increases in salivary IgA and IgG antibody titres in adolescents at one month after vaccination with the conjugate vaccine.5 However, these titres decreased considerably towards baseline within six to 12 months. No studies on antibody titres to Neisseria meningitidis serogroup C in tears after immunisation, or vaccine efficacy in relation to conjunctivitis could be found in the literature.
Vaccination is known to reduce nasopharyngeal carriage of Neisseria meningitidis serogroup C in the community, with protection against carriage of up to 63 per cent after vaccination programs.6 However, carriage is not eliminated in all vaccinated individuals. Extrapolating what is known about the efficacy of the vaccine against nasopharyngeal carriage to the conjunctiva, as another vascular mucosal surface, it is likely that vaccine efficacy against primary meningococcal conjunctivitis is considerably less than that for invasive disease. The point should be made however, that in the absence of invasive disease in the child case, this was not a case of ‘vaccine failure’.
The second issue related to this cluster is the sequence of transmission. The parent case was notified first, but the disease onset occurred two days after the onset of the child’s conjunctivitis. Primary meningococcal conjunctivitis may result from inoculation of the conjunctival sac with meningococci that are either airborne or mechanically transmitted.7 If we assume that the child had pharyngeal colonisation prior to the onset of purulent conjunctivitis, the parent may have acquired meningococci from the child, particularly in light of the child’s ongoing respiratory symptoms at the time. The parent had no contact with the child for the duration of purulent eye discharge, and did not self-report conjunctival symptoms, so mechanical transmission appears less likely.
As the incubation period for invasive meningococcal disease varies from two to seven days, it is possible, although not likely, that meningococci from the parent’s nasopharynx were transmitted to the child whilst she was asymptomatic. The third and most likely possibility, in relation to the sequence of transmission, is that both parent and child acquired meningococci from a common close contact.
The third issue signified in this cluster involves the identification of related cases of disease. While this question is routinely asked during the investigation of meningococcal cases in Queensland, clinicians, patients, their families and public health officers alike assume the question is about other cases of meningitis or septicaemia; invasive disease that causes the symptoms public health messages warn the community about. This cluster highlights a much less distinctive presentation that may be relevant to some cases.
Finally, the issue of contact definitions in relation to meningococcal conjunctivitis is raised by this cluster. Close contacts for prophylaxis have been defined by the Communicable Diseases Network Australia working party on meningococcal disease8 and include contacts of meningococcal conjunctivitis. These guidelines for the control of meningococcal disease do not discuss the possibility of transmission of meningococci from conjunctival exudate.8 Intra and extracellular meningococci have been consistently found in conjunctival exudate.9
Meningococci have also been isolated from the nasopharynx of cases of primary meningococcal conjunctivitis, and have proved to be the same serogroup, serotype and subtype as those isolated from the conjunctiva.10 There are published reports of possible transmission of meningococci to close contacts from cases with primary meningococcal conjunctivitis.9,11 In both cases, the contact was a member of the case’s household. It seems likely, in view of the possibility of organisms colonising the case’s nasopharynx, and the absence of conjunctival symptoms in the contacts, that they acquired meningococci from the cases via droplet spread. However, the possibility exists that meningococci present in conjunctival exudate could also be mechanically transferred to the conjunctiva of a contact, perhaps causing a secondary case of meningococcal conjunctivitis. The incidence of any such phenomenon is not currently known however, and further delineation of the role of conjunctivitis in transmission of Neisseria meningitidis should be sought. Because any secondary case of meningococcal conjunctivitis would have the potential to go on to develop invasive disease7, perhaps future consideration should be given to the provision of specific information about the possibility of mechanical transmission of meningococci to the contacts of a case of primary meningococcal conjunctivitis. If deemed appropriate, this information should recommend contacts seek medical advice if they develop symptoms of conjunctivitis, in addition to being alert for, and seeking medical attention if they develop symptoms of invasive disease.
Top of page
Acknowledgements
The authors gratefully acknowledge Queensland Medical Laboratory and Dr Geoffrey Playford, Infectious Disease Physician, Princess Alexandra Hospital.
References
1. Ramsay ME, Andrews NJ, Trotter CL, Kaczmarski EB, Miller E. Herd immunity from meningococcal serogroup C conjugate vaccination in England:database analysis. BMJ 2003;326:365–366.
2. Ruggeberg J and Heath PT. Safety and efficacy of meningococcal group C conjugate vaccines. Expert Opin Drug Saf 2003;2(1):7–19.
3. MacLennan JM, Shackley F, Heath PT, Deeks JJ, Flamank C, Herbert M, et al. Safety, immunogenicity, and induction of immunologic memory by a serogroup C
meningococcal conjugate vaccine in infants. JAMA 2000;283(21):2795–2801.
4. Richmond P, Borrow R, Miller E, Clark S, Sadler F, Fox A, et al. Meningococcal serogroup C conjugate vaccine is immunogenic in infancy and primes for memory. J Infect Dis 1999;179:1569–1572.
5. Zhang Q, Choo S, Everard J, Jennings R, Finn A. Mucosal immune responses to meningococcal group C conjugate and group A and C polysaccharide vaccines in adolescents. Infect Immun 2000;68(5):2692–2697.
6. Maiden MCJ, Stuart JM. Carriage of serogroup C meningococci 1 year after meningococcal C conjugate polysaccharide vaccination. (research letter). Lancet 2002;359:1829–1830.
7. Barquet N, Gasser I, Domingo P, Moraga FA, Macaya A, Elcuaz R. Primary meningococcal conjunctivitis:report of 21 patients and review. Rev Infect Dis 1990;12(5):838–847.
8. Communicable Diseases Network Australia. Guidelines for the early clinical and public health management of meningococcal disease in Australia. Canberra, Australia:Commonwealth of Australia. 2001.
9. Bigham JM, Hutcheon ME, Patrick DM, Pollard AJ. Death from invasive meningococcal disease following close contact with a case of primary meningococcal conjunctivitis – Langley, British Columbia, 1999. Can Commun Dis Rep 2001;27(2):13–18.
10. Odegaard A. Primary meningococcal conjunctivitis followed by meningitis and septicemia. NIPH Ann 1983;6(1):55–57.
11. Stansfield RE, Masterton RG, Dale BA, Fallon RJ. Primary meningococcal conjunctivitis and the need for prophylaxis in close contacts. J Infect 1994;29(2):211–214.
Top of page
Author affiliations
1. Public Health Registrar, Brisbane Southside Public Health Unit, Brisbane, Queensland
2. Public Health Physician, Brisbane Southside Public Health Unit, Brisbane, Queensland
3. Public Health Microbiology, Queensland Health Scientific Services, Coopers Plains, Queensland
4. Infectious Disease Physician, Princess Alexandra Hospital, Woolloongabba, Queensland
Corresponding author: Dr Megan K Young, Public Health Registrar, Brisbane Southside Public Health Unit, Brisbane, PO Box 333, Archerfield, Queensland 4108. Telephone: +61 7 3000 9148. Facsimile +61 7 3000 9130. Email: megan_young@qld.health.gov.au
This article was published in Communicable Diseases Intelligence Vol 28 No 4, December 2004.
