The National Rotavirus Reference Centre together with collaborating laboratories
Australia-wide has conducted rotavirus surveillance since June 1999. This
report describes the serotypes of rotavirus strains responsible for the hospitalisation
of children with acute gastroenteritis during the period 1 July 2003 to 30
June 2004. We examined 688 faecal samples using monoclonal antibody immunoassays,
reverse transcription-polymerase chain reaction and polyacrylamide gel analysis.
This revealed that serotype G1 has re-emerged as the major serotype nationally,
representing 40 per cent of all strains, followed by serotype G3 (25.7%)
serotype G2 (17.1%) and serotype G9 (11.7%). However, there is substantial
geographic variation in the prevalence of rotavirus serotypes. These findings
have implications for vaccine development strategies which have targeted
prevention of disease due to serotypes G1– G4.
Commun Dis
Intell2004;28:481– 485.
Top of page
Introduction
Group A rotaviruses are the single most important cause of severe gastroenteritis
in young children worldwide. An estimated 400,000– 500,000 children die annually
of severe diarrhoea, however few deaths occur in developed countries.1
Rotavirus induced disease accounts for between 25– 50 per cent of all hospitalisations
for diarrhoea, with 10,000 Australian children hospitalised each year.2
There is wide acceptance of the need for a vaccine to prevent rotavirus disease
in children under five years of age throughout the world, with several vaccines
under development. National rotavirus surveillance provides an understanding
of the epidemiology of rotavirus in Australia, an important component for
success in vaccine development and implementation.
The previous rotavirus surveillance report from the National Rotavirus Surveillance
Program, covering the period July 2002–June 2003, highlighted the potential
importance of uncommon serotypes such as serotype G9. Serotype G9 was first
described in Australia in 19973 and since then
has steadily increased in prevalence to become the dominant serotype nationally
during the 2002/2003 period, representing 74.7 per cent of samples.4
This was the first time since surveillance began in 1993, that serotype G1
was not the dominant type in Australia.
The surveillance and characterisation of rotavirus strains causing annual
epidemics of severe diarrhoea in young children in Australia continues to
be undertaken by the National Rotavirus Reference Centre in Melbourne, together
with seven collaborating centres. In this report we describe the results
of the Australian Rotavirus Surveillance program for the period 1 July 2003
to 30 June 2004, and identify the geographic distribution of the predominant
rotavirus serotypes.
Top of page
Methods
Rotavirus detection was undertaken by enzyme immunoassay (EIA) or latex
agglutination in collaborating laboratories. Rotavirus positive specimens
were collected, stored frozen and forwarded to Melbourne, together with relevant
age and sex details. Specimens were then tested using an in-house monoclonal
antibody (MAb) based serotyping EIA. The EIA employed a panel of MAbs specific
for the major glycoprotein VP7 of the outer capsid of the five major group
A human rotavirus serotypes (G1, G2, G3, G4 and G9).5
Strains which could not be assigned a serotype were genotyped by reverse
transcription/polymerase chain reaction (RT/PCR) using serotype specific
oligonucleotide primers.6 Polyacrylamide gel electrophoresis
(PAGE) was used to classify rotavirus strains genetically into electropherotypes
and to confirm the sharing of the same electropherotype between collaborating
centres.
Top of page
Results
Number of isolates
Six hundred and eighty-eight specimens were received for analysis from Melbourne
and the collaborating centres in Western Australia, Northern Territory and
New South Wales. During the sampling period, no samples were collected from
South Australia, Queensland or Tasmania. Collections in those states resumed
in the financial year beginning July 2004. A total of 608 specimens were
confirmed as rotavirus positive using our in-house EIA assay. Specimens containing
insufficient specimen for testing (n=29) or specimens that were not confirmed
to be positive for rotavirus (n=51) were not analysed further.
Age distribution
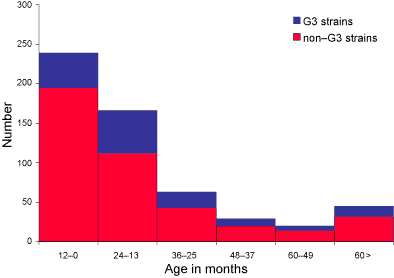
The overall age distribution of the children with acute gastroenteritis
was typical of rotavirus infection (see Figure). In the reporting period,
42.5 per cent of cases were from infants 12 months of age or less, 29.5 per
cent were from patients 13– 24 months of age, and 11.2 per cent were from
patients 25– 36 months of age. Overall, 83.3 per cent of samples were from
children three years or less, and 92 per cent were from children five years
or less. When the age distribution was broken down according to serotypes,
patients aged more than 12 months were significantly more likely to have
a serotype G3 infection (69.9%) than infants aged less than 12 months of
age (30.1% X2 = 11.87, P<0.001, Figure).
Figure. Age distribution versus infecting serotype
Slightly more male children than female were admitted to hospital during
the year, (male to female ratio 1.3:1).
Top of page
Serotype distribution
The rotavirus serotypes identified in Australia from July 1, 2003 to June
30, 2004 are shown in the Table. Serotype G1 was the most common type identified,
representing 40 per cent of all specimens. It was the dominant strain in
only two centres (Melbourne and Sydney), and was identified in six of the
seven centres. Serotype G3 was the second most common serotype nationally
and represented 25.7 per cent of specimens over all. It was identified in
four centres and was the dominant type in Perth and Western Australian Path
centre The two Western Australian centres represent different geographic
locations, one urban (Perth) and one remote, north western Western Australia
(WA Path Centre). Serotype G2 was identified in three centres and represented
17.1 per cent of all specimens. It was the dominant type in Alice Springs.
Serotype G9 was the third most common serotype and represented 11.7 per cent
of all specimens. It was identified in all seven centres, but, was the dominant
type in only two centres (Darwin and Gove). A single serotype G4 isolate
was identified in Melbourne.
During the reporting period, 1.3 per cent of the rotavirus samples analysed
contained multiple serotypes, and in 4.1 per cent of the samples a serotype
was not identified. The latter could be samples with virus numbers below
the detection limits of our assays. Alternatively, these could represent
unusual serotypes not identified using standard methods. For example, we
identified five specimens from Alice Springs which exhibited a super short
RNA electropherotype but were not typeable. Future studies will include further
characterisation of the genes encoding the outer capsid proteins of these
strains.
Rotavirus G serotypes in Australia, 1 July 2003 to 30 June 2004
|
Centre |
Total number |
Serotype percentage (number) |
| G1 |
G2 |
G3 |
G4 |
G9 |
Mixed serotypes |
No result‡ |
| Melbourne |
131 |
86.2
(113) |
0 |
2.3
(3) |
0.8
(1) |
3.8
(5) |
0.8
(1) |
6.1
(8) |
| Sydney |
40 |
75
(30) |
0 |
0 |
0 |
20
(8) |
2.5
(1) |
2.5
(1) |
| Perth* |
148 |
31.1
(46) |
4
(6) |
56.1
(83) |
0 |
5.4
(8) |
2
(3) |
1.4
(2) |
| WA PathCentre* |
137 |
32.1
(44) |
6.6
(9) |
49.7
(68) |
0 |
5.8
(8) |
0.7
(1) |
5.1
(7) |
| Alice Springs |
115 |
4.4
(5) |
77.4
(89) |
0 |
0 |
10.4
(12) |
1.7
(2) |
6.1
(7) |
| Darwin |
33 |
15.1
(5) |
0 |
6.1
(2) |
0 |
78.8
(26) |
0 |
0 |
| Gove |
4 |
0 |
0 |
0 |
0 |
100
(4) |
0 |
0 |
| |
608† |
40
(243) |
17.1
(104) |
25.7
(156) |
0.2
(1) |
11.7
(71) |
1.3
(8) |
4.1
(25) |
Top of page
Discussion
National rotavirus surveillance from 1 July 2003 to 30 June 2004 was highlighted
by the finding that serotypes G1, G2, G3 and G9 were each the dominant type
in at least one of the collaborating centres.
Serotype G1 was the dominant serotype nationally comprising 40 per cent
of all strains. This replaced serotype G9, which was the dominant strain
nationally for the previous two years.4 Serotype
G9 persisted as the dominant strain in Darwin, but not elsewhere in Australia.
Serotype G1, G2 and G3 were the common types in other centres. Serotype G1
was identified in six centres and was the dominant type in Melbourne and
Sydney and the second most common serotype in two other centres Perth and
WA Path centre. The re-emergence of serotype G1 as the dominant strain reinforces
the importance of this serotype. G1 was the dominant serotype in surveys
conducted in Australia from 1993 to 19967, and
during the 1999/2000 and 2000/2001 surveys.8,9
These findings are supported by epidemiological studies conducted throughout
the world which have continued to identify serotype G1 as the dominant serotype.10– 14
The decline in prevalence of serotype G9 has been as dramatic as its emergence.
Serotype G9 was first identified during Australia-wide surveillance in 1997,3
and became the second most prevalent serotype nationally during the 1999/2000
and 2000/2001 surveys, representing 10 per cent and 18.1 per cent respectively
of specimens collected in those years.8,9 G9 became
the dominant strain nationally in 2001/2002, comprising 40 per cent of the
strains15 and 2002/2003 comprising 74.7 per cent.4
However, during the current survey, G9 while present in each centre, represented
only 11.7 per cent of all strains. The decline in the prevalence of G9 strains
around Australia in 2003/2004 was associated with an increase in the prevalence
of G1 and G3 strains.
The increase in prevalence of serotype G3 in Australia has been dramatic.
During the four previous surveys conducted Australia-wide, (1999/2000, 2000/2001,
2001/2002 and 2002/2003) serotype G3 represented less that two per cent of
all strains. However during this survey, the prevalence of serotype G3 has
increased to 25.7 per cent and was the dominant strain in West Australia.
The high prevalence of G3 in Australia is remarkable when compared with the
low prevalence rates of this serotype reported in other countries.10– 14
Interestingly, the emergence of serotype G3 has also been recently identified
in two regions (Qinhuangdao and Zheng zhou) in a recent study from China,16
and represented 45 and 80 per cent of isolates from each region. Whether
these G3 strains move eastward from WA to Sydney and Melbourne, and have
an Australia-wide impact similar to serotype G9, will be followed with interest
during the next rotavirus season. The increase in prevalence of serotype
G3 appears to have been associated with changes in the age distribution of
children infected with rotavirus, when compared to non-G3 strains. While
the majority of children (87%) infected with rotavirus were under three years
of age, the G3 strains infected children aged 13– 24 months more frequently
than children aged 12 months or less (p<0.001). In contrast, over 50 per
cent of the children infected with the other rotavirus serotypes were under
12 months of age. This data suggests that pre-existing antibodies may not
protect against subsequent severe re-infection with the serotype G3 strain.
An outbreak of severe gastroenteritis again swept through Alice Springs
in Central Australia causing a major impact on health care facilities in
January 2004. Serotype G2 was responsible for this year’s outbreak. The previous
G2 outbreak in Alice Springs in 1993, was shown to be due to an unusual G2
strain derived by reassortment between subgroup I and subgroup II human strains.17
The 2004 strain possessed the standard short pattern electropherotype and
subgroup I antigenicity. Serotype G2 strains have previously been responsible
for intermittent epidemics in several of the other centers during the past
12 years, including in Perth in 1993, Melbourne in 1994, and Sydney
in 2001.7,9
These results together with those of previous years highlight the continuing
change in the prevalence and emergence of new rotavirus serotypes. Multi-centre
surveillance of rotavirus is important to continue to monitor strains in
Australia. These results contribute to worldwide knowledge of rotavirus epidemiology
and essential to inform the development of new rotavirus vaccines.
Top of page
Acknowledgements
The Rotavirus Surveillance program is supported by grants from the Australian
Government Department of Health and Ageing, GlaxoSmithKline and CSL. Dr Kirkwood
is supported by The Phillip Bushell Research Fellowship awarded by the Gastroenterological
Society of Australia.
Rotavirus positives were collected from numerous centres throughout Australia.
The significant time and effort involved in the collection, storage, packaging,
compiling data and forwarding of specimens was much appreciated. Without
the contribution of the following people the study would not have been possible.
Western Australia
Dr K Lindsay and members of the Virology Department, Princess Margaret Hospital
for Children, Subiaco, WA 6008.
Dr D Smith, Dr G Harnett and members of Division of Microbiology, Path Centre.
The Queen Elizabeth Medical Centre, Nedlands WA, 6009.
Northern Territory
J De Boer and members of the Microbiology Department, Royal Darwin Hospital,
Casuarina, NT 0810.
B Truscott and members of the Pathology Department, Western Diagnostic Pathology,
Tiwi, NT, 0810.
F Morey and members of the Microbiology Department, Alice Springs Hospital,
Alice Springs, NT, 0971.
K Carter and members of Pathology Department, Gove District Hopsital, Nhulunbuy,
NT 0880.
New South Wales
W Rawlinson and C McIver and members of the Virology Division, prince of
Wales Hospital, NSW, 2031.
Victoria
Dr R Schnagl, School of Microbiology, La Trobe University, Bundoora, Vic,
3083.
Dr R Alexander and members of the Virology Department, Royal Children’s
Hospital, Parkville, Vic, 3052.
Top of page
References
1. Parashar U, Hummelman
E, Bresee J, Miller M, Glass R. Global illness and deaths caused by rotavirus
disease in children. Emerg Infect Dis 2003;9:565– 572.
2. Carlin J, Chondros P,
Masendycz P, Bugg H, Bishop R, Barnes G. Rotavirus infection and rates of
hospitalisation for acute gastroenteritis in young children in Australia,
1993– 1996. Med J Aust 1998;169:252– 1256.
3. Palombo E, Masendycz
P, Bugg H, Bogdanovic-Sakran N, Barnes G, Bishop R. Emergence of serotype
G9 human rotaviruses in Australia. J Clin Microbiol
2000;38:1305– 1306.
4. Kirkwood C, Bogdanovic-Sakran
N, Clark R, Masendycz P, Bishop R, Barnes G. Report of Australian rotavirus
surveillance program, 2002/2003. Commun Dis Intell
2003;27:492– 1495.
5. Coulson BS, Unicomb LE,
Pitson GA, Bishop RF. Simple and specific enzyme immunoassay using monoclonal
antibodies for serotyping human rotaviruses. J Clin Microbiol
1987;25:509– 515.
6. Gouvea V, Glass R, Woods
P, Taniguchi K, Clark H, Forrester B, et al. Polymerase chain reaction amplification
and typing of rotavirus nucleic acid from stool specimens. J
Clin Microbiol 1990;28:276– 282.
7. Bishop R, Masendycz P,
Bugg H, Carlin J, Barnes G. Epidemiological patterns of rotavirus causing
severe gastroenteritis in young children throughout Australia from 1993 to
1996. J Clin Microbiol2001;39:1085– 1091.
8. Masendycz P, Bogdanovic
N, Palombo E, Bishop R, Barnes G. Annual report of the rotavirus surveillance
Program, 1999/2000. Commun Dis Intell 2000;24:195– 198.
9. Masendycz P, Bogdanovic
N, Kirkwood C, Bishop R, Barnes G. Annual report of the rotavirus surveillance
Program, 2000/2001. Commun Dis Intell. 2001;25:143– 146.
10. Gentsch J, Woods P, Ramachandran
M, Das B, Leite J, Alfieri A, et al. Review of G and P typing results from
a global collection of rotavirus strains; implications for vaccine development.
J Infect Dis 1996;174:S30– S34.
11. Banyai K, Gentsch J, Glass R, Uj
M, Mihaly I, Szucs G. Eight-year survey of human rotavirus strains demonstrates
circulation of unusual G and P types in Hungary. J Clin Microbiol
2004;42:393– 397.
12. Bon F, Fromantin C, Aho S, Pothier
P, Kohli E. G and P genotyping of rotavirus strains circulating in France
over a three-year period:detection of G9 and P6 strains at low frequencies.
The AZAY Group. J Clin Microbiol 2000;38:1681– 1683.
13. Griffin D, Kirkwood C, Parashar
U, Woods P, Bresee J, Glass R, et al. Surveillance of rotavirus strains in
the United States:identification of unusual strains. J Clin Microbiol
2000;38:2784– 787.
14. Iturriza-Gomara M, Green J, Brown
D, Ramsay M, Desselberger U, Gray J. Molecular epidemiology of human group
A rotavirus infections in the United Kingdom between 1995 and 1998. J
Clin Microbiol 2000;38:4394– 4401.
15. Kirkwood C, Bogdanovic-Sakran N,
Clark R, Masendycz P, Bishop R, Barnes G. Report of Australian rotavirus
surveillance program, 2001/2002. Commun Dis Intell
2002;26:537– 540.
16. Fang Z, Yang H, Qi J, Zhang J, Sun
L-W, Tang J-Y, et al. Diversity of rotavirus strains among children with
acute diarrhoea in China:1998– 2000 surveillance study. J Clin
Microbiol 2002;40:1875– 1878.
17. Palombo E, Bugg H, Masendycz P,
Coulson B, Barnes G, Bishop R. Multiple-gene rotavirus reassortants responsible
for an outbreak of gastroenteritis in central and northern Australia. J
Gen Virol 1996;77:1223– 227.
Top of page
Author affiliation
Corresponding author: Carl Kirkwood, National Rotavirus Reference Centre, Murdoch Children’s Research Institute, Royal Children’s Hospital, Flemington Road, Parkville, Victoria, 3052.
This article was published in Communicable Diseases Intelligence Vol 28 No 4, December 2004.
