This paper reports the clinical features and outcome of all children with a laboratory proven diagnosis of influenza A virus infection admitted to a major Paediatric Intensive Care Unit (PICU) in 2003. Eight of the 22 patients with influenza A virus infection (A/Fujian/411/2002-like type) presented with encephalopathy and three of the 22 patients died. This can be compared with 44 admissions and seven (16%) deaths of patients with influenza virus admitted in the same PICU in the preceding 15 years. In the present cohort, four (18%) of the 22 patients, including one child who died, should have received influenza vaccine according to the current Australian immunisation recommendations. We have no documented evidence that any of the 22 children received influenza vaccination. During the 2003 influenza season there was an increased number of children admitted to our PICU with influenza A infection and an increased number of deaths compared with previous years. Influenza infection causes significant morbidity and mortality in young children, most of whom are not currently recommended for annual influenza vaccination. Commun Dis Intell 2004;28:504–509.
Top of page
Introduction
Influenza is a common disease of childhood with the highest morbidity and mortality occurring in preschool aged children.1,2,3 In Australia between 1 July 1998 and June 2000 the rate of hospitalizations of children aged 0–4 years with influenza infection was 70.6/100,000 population, far exceeding rates in all other age groups. The number of deaths attributed to influenza in children are 12/100,000 and 2/100,000 for 0–4 year olds and 5–12 year olds respectively.3 Annual influenza immunisation is currently recommended in the Australian Immunisation Handbook for children at risk of severe influenza, but not routinely for healthy children.4 However, recent reports of increased morbidity and mortality in children with influenza associated illnesses in North America has led to updating the United States of America (USA) recommendations for influenza vaccination to include all healthy children six–23 months during the influenza season to the extent that is logistically and economically feasible.5, 6
In this study we describe the experience of a tertiary children’s hospital Intensive Care Unit in Sydney, during the 2003 influenza season. The Children’s Hospital at Westmead is one of two Sydney paediatric teaching hospitals which serve New South Wales, with a current population of 6.7 million people. The PICU is a 23 bed intensive care unit which has over 1,000 admissions each year. Of this number, 25–30 per cent are admitted with general medical problems. Almost 70 per cent of the PICU admissions are from the Sydney metropolitan area, the remainder from rural areas.
Top of page
Methods
We reviewed the medical records of all patients identified with laboratory-proven influenza A virus infection admitted to the PICU during 2003. We compared the 2003 outbreak with the number of admissions and deaths of patients in PICU with laboratory proven influenza virus infection over the last 15 years. Ethical approval for this study was obtained from the hospital ethics committee. Patients were identified by reviewing the Intensive Care Unit database and virology records from 1988–2002. Data collected from the 2003 cohort of patients included date of birth, age, sex, underlying medical condition, clinical presentation, diagnosis, source of isolation of influenza, other positive cultures, vaccination status if documented, length of stay in PICU and the outcome. These patients were compared with groups targeted for influenza immunisation according to the current Australian Immunisation Handbook.4
A laboratory proven case was defined as a child with influenza virus identified either by direct immunoflourescence (DFA) or from viral culture from a nasopharyngeal aspirate (NPA) or by a 4-fold rise in influenza antibody titre in paired sera. Routine practice in the PICU is that all children admitted with respiratory symptoms or possible viral infections are tested for viral antigens on a NPA and viral culture. In our hospital NPAs are tested for respiratory syncytial virus (RSV), parainfluenza viruses 1, 2 and 3, influenza viruses A and B and adenoviruses using DFA (Imagen kit by Dako). Isolates with positive DFAs for an influenza virus are then cultured. Nasopharyngeal aspirates with negative DFAs were also routinely set up for viral culture for three weeks. Cultured isolates were sent to the World Health Organization Collaborating Centre for Reference and Research on Influenza, in Melbourne, Australia for typing by haemagglutination inhibition assay.
Top of page
Results
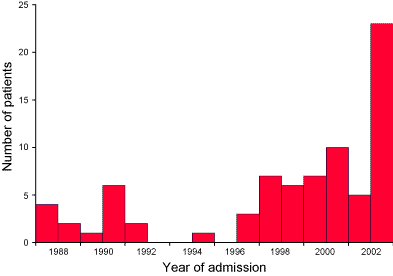
Twenty-two patients (23 admissions) with laboratory-proven influenza A virus were admitted to the Intensive Care Unit in 2003. This was five per cent of the total medical admissions to PICU in 2003. The virus strain was isolated as Influenza A (H3N2) of the Fujian/411/2002-like type. Three (14%) patients died. All admissions occurred during a 3 week period in August, except for one patient who was admitted in late September. In comparison, over the 15 years from 1988 to 2002, there were 44 PICU admissions and seven (16%) deaths of children with laboratory proven influenza infection (Figure).
Figure. Number of patients admitted to Paediatric Intensive Care Unit with influenza associated illness between 1988 and 2003
Clinical features of the 22 patients admitted to PICU with influenza A infection are set out in the table. Patients are listed in order of admission. The mean age in this cohort was 4.1 years (range 4 months–13 years) of whom 16 (73%) patients were under four years of age, six (27%) between six and 23 months of age and one under six months of age. Of the 22 patients there were 12 (55%) males and 10 (45%) females. Eleven (50%) of the patients had a pre-existing condition, including two of the three patients who died.
Table. Clinical features of patients with influenza associated illness admitted to Paediatric Intensive Care Unit in 2003
| n |
Sex |
Age years |
Pre-existing morbidity |
Diagnosis |
LOS. p |
LOS.h |
Outcome |
| 1 |
F |
3 |
DD/ Seizures |
Pneumonia/Seizures |
4.6 |
6 |
d/c |
| 2 |
F |
8 |
Seizures/GOR |
Septic shock |
2.3 |
7.5 |
Died |
| 3 |
M |
1.4 |
CHD |
Pneumonia |
9.1 |
10.8 |
d/c |
| 4 |
M |
2.3 |
Nil |
Laryngotracheobronchitis |
2.1 |
4.1 |
d/c |
| 5 |
M |
2 |
Nil |
Laryngotracheobronchitis |
1.5 |
2.3 |
d/c |
| 6 |
F |
3 |
Liver disease |
Bleeding Varices |
0.8 |
10.5 |
d/c |
| 7 |
M |
1.9 |
Nil |
Meningococcal septicaemia |
6.5 |
9.9 |
d/c |
| 8 |
M |
0.75 |
CLD |
Pneumonitis |
0.7 |
4.8 |
d/c |
| 9 |
F |
4 |
Nil |
Cardiac Arrest |
4.8 |
4.8 |
Died |
| 10 |
M |
0.8 |
Nil |
Bronchiolitis/Pneumonia |
1 |
5.5 |
d/c |
| 11 |
M |
7 |
CP hemiparesis |
Encephalopathy/Pneumonitis |
1.3 |
1.3 |
Died |
| 12 |
F |
8 |
Nil |
Pneumonitis/Pneumothorax |
2.8 |
4.1 |
d/c |
| 13 |
M |
13 |
Mild DD |
Pneumonia/Pancreatitis/Encephalopathy |
11.4 |
27.3 |
r/a |
| 14 |
M |
8 |
Nil |
Meningoencephalitis |
1.1 |
2.5 |
d/c |
| 15 |
F |
0.4 |
DD/CAH |
Status Epilepticus |
1.7 |
11 |
d/c |
| 16 |
F |
1.1 |
Nil |
Pneumonitis/Asthma |
0.7 |
8.6 |
d/c |
| 17 |
M |
2.8 |
Nil |
Pneumonia/Empyema |
0.7 |
15.5 |
d/c |
| 18 |
F |
3 |
Asthma |
Asthma |
0.8 |
3.8 |
d/c |
| 19 |
F |
2.6 |
Nil |
Encephalopathy/ICH |
7.1 |
25 |
d/c |
| 20 |
M |
5 |
Asthma |
Encephalitis/Seizures |
0.7 |
4.5 |
d/c |
| 21 |
M |
1.8 |
Nil |
Pneumonia/Hepatoblastoma |
8.9 |
16.5 |
d/c |
| 22 |
F |
2.6 |
Asthma |
Gastroenteritis/Encephalopathy |
0.6 |
4 |
d/c |
All patients presented with fever and 15 (68%) with clinical evidence of either an upper respiratory tract infection or lower respiratory tract infection defined as tachypnoea, cough, wheeze or respiratory distress. Eight (36%) patients presented with encephalopathy, defined as altered mental status of any duration, including seizures but not including simple febrile convulsions.
During admission, pneumonia or pneumonitis was reported in 10 (45%) patients. One patient had Gram negative diplococci on skin scraping and clinical features of meningococcal septicaemia concurrent with influenza infection. There were no patients with positive blood cultures. The mean length of stay in PICU was 3.2 days (range 0.6 to 11.4 days) compared with the mean length of stay for all patients in this unit of 3.1 days.
Influenza A was detected by DFA from NPAs in all patients. There were no isolates of influenza B virus. Influenza A virus was recovered from 14 samples, all which were positive for influenza A (H3N2) of the Fujian/411/2002-like type.
According to the 7th edition of the Australian Immunisation Handbook,4 routine annual influenza immunisation was recommended for four (18%) of the 22 patients including one patient who died. There was no documented evidence that any of the patients were vaccinated against influenza.
Top of page
Case reports
Clinical details of the three patients who died are presented as brief case reports below.
Patient 1
A four-year-old girl, who was previously well, developed coryza, lethargy and rash 48 hours prior to presentation. She was seen by the local doctor who prescribed oral penicillin. She became increasingly lethargic and collapsed at home. Cardiopulmonary resuscitation was commenced by her father and continued until the ambulance arrived. At this time she had no respirations and was pulseless. She was intubated, ventilated, given endotracheal and intravenous adrenaline and atropine after which her circulation returned. On arrival at the emergency department she had evidence of haemodynamic shock, a Glasgow Coma Score of 4 and temperature of 36.2oC. The chest x-ray (CXR) showed bilateral infiltrates. She was transferred to PICU and subsequently developed evidence of acute lung injury. Influenza A was isolated from NPA collected on day 1 of admission. Her respiratory state improved over the initial 24 hours but she developed signs of cerebral oedema and at 72 hours of the admission, brain stem testing revealed brain death. No other pathogens were isolated. Post mortem was not performed.
Patient 2
An eight-year-old girl with a background of severe developmental delay, seizures, gastroesophageal reflux and gastrostomy feeds presented to hospital with a 4-day history of fever, cough and respiratory distress. She was admitted to intensive care after worsening in her respiratory status requiring intubation and ventilation. Her CXR showed prominent interstitial markings with right lower lobe consolidation. Influenza A was detected from an NPA collected on day 1 of admission. She required artificial ventilation, went on to develop haemodynamic shock, intractable multi-organ failure and died. Post mortem was not performed.
Patient 3
A seven-year-old boy with a background of right sided hemiparesis from congenital cerebral atrophy presented with a 48 hour history of cough, coryza and vomiting. He was only mildly affected by his hemiparesis, being able to ride a bike and attend a normal school. After feeling unwell at midday, he went to rest and was found obtunded seven hours later. On arrival at the emergency department his Glasgow Coma Score was 3 and temperature was 35oC. He was intubated, ventilated, commenced on intravenous cefotaxime, acyclovir and transferred to PICU. CXR showed opacification of the right lung field and patchy consolidation of the left. Influenza A was detected from NPA collected on day 1 of admission. Electroencephalogram showed encephalopathic changes. A magnetic resonance imaging scan of his brain showed widespread cerebral oedema, ischaemia and bilateral uncal and transtentorial herniation. Brainstem testing confirmed brain death. Post mortem was not performed.
Top of page
Discussion
Influenza infections cause substantial morbidity and mortality in children every year.3,7 Our data have shown an increase in admissions to PICU and an increased number of deaths of children with influenza associated illnesses in 2003 compared to the past 15 years and with a similar study of admissions from 1974–1994.8 We believe that influenza A infection was the principal cause of mortality and acute morbidity in our 22 patients. One patient admitted with laboratory-proven influenza infection also had meningococcal septicaemia. Previous reports have shown an association between outbreaks of influenza and meningococcal disease.9 Whilst we do not have post mortem data in those patients who died, we have no other identified cause of their admitting illness despite intensive anti-mortem investigations. The New South Wales Influenza Surveillance Annual Report states that the 2003 influenza season was moderate,10 suggesting that the increase in paediatric disease severity was out of keeping with the overall experience.The National Notifiable Diseases Surveillance System reported in comparison to 2002, notification rates of influenza declined in the over 65 age group but increased among the 0–4 year age group and remained unchanged in the rest of the age groups.11 The majority of influenza A isolates identified during the peak period was A (H3N2) viruses of the A/Fujian/411/2002 type.10 This same strain was isolated in the cohort of patients described in this paper.
Thirty-six per cent of our patients presented with encephalopathy associated with influenza illness. This finding is similar to reports of the 2003 Michigan influenza season, when surveillance identified four deaths and 10 severe illnesses among children and adults less than 21 years with influenza associated illness. Eight (57%) of these 14 cases, had evidence of encephalopathy and one had evidence of myocarditis; two of the children with encephalopathy died.5 Similarly, in Virginia, five unexplained deaths associated with influenza infection in children aged two to seven years were reported in the 2003 influenza season.12 An increased number of cases of influenza-associated encephalopathy in children less than five years of age was also been reported from Japan.13,14,15 The Centres for Disease Control in the USA recently published a report of 142 influenza associated deaths among children less than 18 years in their current winter season as of 27th March 2004.6 Over half of these deaths were in children under five years of age, and only 21 had high-risk medical conditions that put them at risk for complications of influenza.16
In Australia, although influenza hospitalization rates in children aged 0–4 years far exceed the rates in all other age groups,3 the only children targeted for annual influenza immunisation include those with chronic cardiac or pulmonary disorders, children residing in chronic care facilities, and children with chronic conditions such as diabetes mellitus and other metabolic diseases, cancer, immunodeficiency, immunosuppression, renal disease, anaemia and haemoglobinopathy.4 A recent study in Melbourne reported the impact of influenza A (H3N2) during the 2003 outbreak on children with disabilities with an increased hospital admission rate, PICU admission and length of stay.17 However over 80 per cent of patients in our cohort and two of the three patients who died, did not belong to the specific high-risk groups currently recommended for annual influenza vaccination. Similarly, a study by Quach et al in Montreal Children’s Hospital found that the majority of children hospitalised for influenza did not belong to specific risk groups targeted by current recommendations and one third were less than six months of age, hence too young to be vaccinated.1
In 2003 antigenic drift was detected in the H3N2 virus strains circulating in Australia and New Zealand.10 The A/Fujian-like virus is related to the A/Moscow-like strain included in the 2003 vaccine. Perhaps this A/Fujian virus caused an increase in the influenza associated childhood morbidity and mortality throughout the country which will be apparent when the national data are available for 2003. If so, a similar pattern of illness could be predicted in 2004 and beyond.
There are relatively few data on the efficacy of inactivated influenza vaccine in the paediatric population.18 Even though two doses at least one month apart are recommended for children aged less than nine years who are receiving influenza vaccine for the first time,4 it is possible that the cost effectiveness of this vaccine would compare favorably with the vaccination program in this age group including conjugated pneumococcal and meningococcal vaccines.
In response to the increased morbidity and mortality of influenza associated illnesses in the USA, the Advisory Committee on Immunization Practices and the American Academy of Pediatrics now recommend influenza immunisation of all healthy children who will be six to 23 months of age during the influenza season as well as encourage vaccination of household contacts and out-of-home caregivers of children younger than 24 months of age.19,20,21 This decision is based on the higher hospitalization rates within this age group. These new recommendations however may not have changed the outcome of most of the deaths previously described in the USA nor indeed the four, seven and eight year old that died in our cohort. Delivery of inactivated influenza vaccine on an annual basis to Australian children under the age of 23 months would be difficult and could potentially compromise the existing recommended schedule. Despite these difficulties, we believe that in view of our experience in 2003, Australia should now seriously reconsider recommendations for influenza vaccination to include not only children at risk of severe complications but also healthy children. Should we follow the American guidelines and recommend vaccination of all healthy six to 23 month olds, or should we extend this to 48 months in order to cover the age most affected by influenza associated illnesses? In the mean time, we must improve coverage by actively encouraging paediatricians and local doctors to vaccinate children who belong to risk groups according to the current Australian recommendations.
Undoubtedly, heightened awareness of the severe complications and deaths associated with influenza among children is necessary. Mandatory notification of influenza associated deaths among children, as in the USA, needs to be considered to provide more immediate surveillance of the severity of influenza outbreaks.20 In reporting this cohort of children, we aim not only to emphasis the need to provide annual vaccination to children in high-risk groups, but also to stimulate discussion regarding change in Australian vaccination recommendations to include all healthy children aged 6–23 months.
Top of page
Acknowledgements
The authors would like to thank Professor David Isaacs for reviewing this manuscript and the World Health Organization Collaboratory Centre for Reference and Research (Melbourne) for typing the patients’ isolates.
Top of page
References
1. Quach C, Piche-Walker L, Platt R, Moore D. Risk factors associated with severe influenza infections in childhood: implication for vaccine strategy. Pediatrics 2003;112:197–201.
2. Neuzil KM, Zhu Y, Griffin MR, Edwards KM, Thompson JM, Tollefson SJ, et al. Burden of interpandemic influenza in children younger than 5 years: A 25-year prospective study. J Infect Dis 2002;185:147–152.
3. McIntyre P, Gidding H, Gilmour R, Lawrence G, Hull B, Horby P, et al. National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases. Vaccine preventable diseases and vaccination coverage in Australia, 1999 to 2000. Commun Dis Intell 2002;26 Suppl:i–xi:1–111.
4. Australian Immunisation Handbook. 7th Edition Canberra: National Health and Medical Research Council; 2000.
5. CDC. Severe morbidity and mortality associated with influenza in children and young adults - Michigan, 2003. MMWR Morb Mortal Weekly Rep 2003;52:837–840
6. CDC. Update: Influenza Activity—United States, 2003–04 season. MMWR Morb Mortal Weekly Rep 2004;53:284–287.
7. Thompson WW, Shay DK , Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003;289:179–186.
8. Kappagoda C, Isaacs D, Mellis C, Peat, J, De Silva L, O’Connell A. Critical influenza virus infection. J Paediatr Child Health 2000; 36, 318–321.
9. Cartwright KA, Jones DM, Smith AJ, Stuart JM, Kaczmarski EB, Palmer SR. Influenza A and Meningococcal disease. Lancet 1991;338:554–557.
10. NSW Influenza Surveillance Annual Report. NSW Department of Health Report No 23: 5 May to 11 October 2003. Report date: 10 November 2003.
11. Yohannes K, Roche P, Hampson A, Miller M, Spencer J. Annual report of the National Influenza Surveillance Scheme, 2003. Commun Dis Intell 2004;28:160–168.
12. Oklahoma Influenza Surveillance 2002–2003. Oklahoma State Department of Health Report for Week Ending March 7, 2003.
13. Nikaido K, Agatsuma Y, Inoue M, Ohara T, Nihira H, Wakai S, et al. Delayed onset encephalopathy associated with influenza A virus infection. Pediatr Infect Dis J 2003;22:849–851.
14. Kasai T, Togashi T, Morishima T. Encephalopathy associated with influenza epidemics. Lancet 2000;355:1558–1559.
15. Morishima T, Togashi T, Yokota S, Okuno Y, Miyazaki C, Tashiro M et al. Encephalitis and encephalopathy associated with an influenza epidemic in Japan. Clin Infect Dis 2002;35:512–517.
16. CDC. Update: Influenza Activity—United States, January 11–17, 2004. MMWR Morb Mortal Weekly Rep 2004;53:35–37.
17. Stark ZL, Buttery JP, Antolovich GC, Reddihough DS. The Impact of Influenza A on Children with Disabilities. J Paediatr Child Health 2004;40,332.
18. Zangwill KM, Belshe RB. Safety and efficacy of trivalent inactivated influenza vaccine in young children: a summary for the new era of routine vaccination. Pediatr Infect Dis J 2004;23:189–197.
19. Baltimore RS, Jenson HB. New recommendations for influenza vaccination for children and pregnant women. Current Opinion Pediatrics 2003;15:74–76.
20. CDC. Update: Influenza-associated deaths reported among children aged <18 years—United States, 2003–2004 influenza season. MMWR Morb Mortal Weekly Rep 2004;52:1286–1288.
21. American Academy of Pediatrics Committee on Infectious Diseases. Recommended Childhood and Adolescent Immunization Schedule– United States, July– December 2004. Pediatrics 2004:113:1441–1448.
22. CDC. Update: Influenza activity&mdashUnited States and worldwide, 2002–03 season, and composition of the 2003–04 influenza vaccine. MMWR Morb Mortal Weekly Rep 2003: 52:516–512.
Top of page
Author affiliations
1. Paediatric Intensive Care Unit, The Children’s Hospital at Westmead, Westmead, New South Wales
2. The Department of Microbiology and Virology, The Children’s Hospital at Westmead, Westmead, New South Wales
3. The National Centre for Immunisation Research, The Children’s Hospital at Westmead, Westmead, New South Wales
Correspondence author: Dr Bronwyn Milne, The Children’s Hospital at Westmead, Locked Bag 4001, Westmead, NSW 2145. Telephone: +61 2 9845 0000. Facsimile; +61 2 9845 0200. Email: bronwym2@chw.edu.au
This article was published in Communicable Diseases Intelligence Vol 28 No 4, December 2004.
