Michael Watson,1
Paul Roche,2
Kathy Bayley,5
Jan M Bell,3
Peter Collignon,4
Gwendolyn L Gilbert,5
Geoff Hogg,6
Anthony D Keil,7
Vicki Krause,8
Denise Murphy,9
Helen V Smith,9
Mitchell Brown,1
Joanne Stylianopoulos,6
John Turnidge3
Introduction | Methods | Results | Discussion | Acknowledgements | References
Abstract
A comprehensive invasive pneumococcal disease (IPD) laboratory surveillance program was carried out in Australia in 2003. This program provided data on the prevalence of pneumococcal serotypes and antimicrobial resistance.
There were 1,995 isolates tested with 34 per cent (683) from children aged
less than five years and 27 per cent (535) from the elderly aged more than
65 years. One thousand eight hundred and sixty were isolates from blood,
79 from CSF and 56 from other sterile sites. In young children, 84 per cent
of isolates were a serotype and 92 per cent a serogroup in the 7-valent pneumococcal
conjugate vaccine (7vPCV). Of penicillin resistant isolates in children less
than five years of age 85 per cent and 98 per cent were a serotype and serogroup
in the 7vPCV respectively. When the universal 7vPCV vaccine program in young
children is introduced in 2005, a proportion of cases of IPD should also
be prevented in young adults (estimated reduction of 54 cases annually) and
elderly Australians (an estimated reduction of 110 cases annually) as a result
of improved herd immunity. Pneumococcal serotypes with higher rates of penicillin
resistance (19F, 14 and 6B) were more prevalent in the elderly than in young
children. In contrast, erythromycin resistance was more common in children
less than five years of age (24%) compared to the elderly (15%). The predominant
serotype with erythromycin resistance in Australia was serotype
14 and thus there is likely to be a major reduction in erythromycin resistance
as a result of 7vPCV vaccination. Continued surveillance of pneumococcal
serotype distribution and antibiotic susceptibility will be essential in
order to identify serotype replacement by non-vaccine serotypes and to monitor
the overall impact of current and future vaccine programs on invasive pneumococcal
disease in Australia, not only in young children but also
in other age groups.
Commun Dis Intell
2004;28:455 464.
Top of page
Introduction
Streptococcus pneumoniae
is a major cause of morbidity and mortality world-wide.1–4
It is a common cause of life threatening invasive disease (e.g. bactaeremia
and meningitis) as well as non invasive disease (e.g. otitis media). It is
important that comprehensive laboratory surveillance of invasive pneumococcal
disease (IPD) is undertaken to assess the success of universal childhood
7-valent conjugate pneumococcal (7vPCV) vaccination which will be implemented
in Australia in 2005.
Laboratory surveillance of IPD has been conducted in various Australian states
and territories prior to 2002.5–9 This report
summarises the results of laboratory surveillance for all Australian jurisdictions
in 2003 and includes comprehensive pneumococcal serotyping of these isolates
and antimicrobial resistance data.
Antimicrobial resistance in invasive pneumococci is an emerging problem
in Australia.10 Laboratory
data on resistance to penicillin and erythromycin and the key serotypes responsible
for antimicrobial resistance in each state and territory are reported. The
potential benefits of the universal childhood immunisation program for adults
are discussed.
Top of page
Methods and Materials
Case definition
For the purposes of laboratory surveillance, a case of IPD was included
when Streptococcus pneumoniae
was isolated by culture from a normally sterile body site (blood, cerebrospinal
fluid (CSF), joint fluid etc). Only one isolate was tested from each patient
episode. A new episode was deemed to occur if an isolate was cultured more
than 14 days after a previous positive culture.
Data sources and collection
A network of laboratories in Australia (see
list of participating laboratories) obtained pneumococcal isolates referred
from all major private and public microbiology laboratories in Australia. Isolates
were stored for later serotyping at one of the three designated pneumococcal
typing laboratories. Indigenous status data was linked to laboratory data
only in the Northern Territory in 2003 and detailed analysis by Indigenous
status was not performed in this year’s report in contrast to the 2002 report.11
Enhanced data on IPD including information on pneumococcal serotypes in Indigenous
people are collected as an extension of the National Notifiable Diseases
Surveillance System (NNDSS) and the 2003 data are provided in the accompanying
surveillance report.12
Serotyping
Pneumococcal serotyping was performed at the Pneumococcal Reference Laboratory
of Queensland Health Scientific Services (for Western
Australia, Northern Territory and Queensland),
the Children’s Hospital at Westmead’s NSW Pneumococcal Reference Laboratory
(for New South Wales and the Australian
Capital Territory) and the Microbiological Diagnostic Unit (for Victoria, Tasmania and South Australia). Serotyping was performed by
the Quellung reaction using antisera from the Statens Seruminstitut, Copenhagen,
Denmark.13
Analysis of serotypes included the prevalence of vaccine serotypes and
vaccine serogroups (that is, pneumococci with serotypes within the same serogroups
as vaccine types).14
The pneumococcal serotypes in the three vaccines referred to in this paper
are shown in Table 1.
Table 1. Pneumococcal vaccines and constituent serotypes referred to in this report
| Vaccine |
7-valent conjugate vaccine (7vPCV) |
11-valent conjugate vaccine (11vPCV) |
23-valent polysaccharide vaccine
(23vPPV) |
| PneumococcalSerotypes |
4, 6B, 9V, 14, 18C, 19F, 23F |
1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, 23F |
1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, 33F |
Susceptibility testing
Susceptibility testing was performed by a range of different methods.
In New South Wales, Victoria, Tasmania, Australian
Capital Territory and South Australia
the available results were from routine diagnostic laboratories. These laboratories
used National Committee for Clinical Laboratory Standards (NCCLS) disc diffusion,15
Calibrated Dichotomous Susceptibility (CDS) disc diffusion16
or agar dilution susceptibility testing methods. Most laboratories also confirmed
penicillin resistance using the E test method.17
Results from Queensland, Northern Territory and Western Australia were performed
using NCCLS disc diffusion and E test methods in a reference laboratory.
Isolates were categorized as fully sensitive to penicillin or resistant
(includes intermediate and high level resistance using NCCLS breakpoints
(Minimum Inhibitory Concentration (MIC) ≥0.12). Erythromycin was categorized
as either sensitive or resistant (MIC ≥1mg/L).
Statistical analysis
Yates corrected Chi square test was used for univariate analysis using
Epi info statistical software Version 6.02 (CDC, USA).
Top of page
Results
Cases under laboratory surveillance
There were 1,998 pneumococcal isolates forwarded to the three pneumococcal
reference laboratories for serotyping and 1,995 were successfully serotyped.
This represents 92 per cent of the 2,174 notified cases of invasive pneumococcal
disease in Australia in
2003.12 The number of
isolates by state and territory and specimen type is shown in Table 2.
Table 2. Pneumococcal isolates analysed in this report, by reporting jurisdiction and specimen type
| |
Blood |
Cerebrospinal fluid |
Other sites* |
Total |
| ACT |
45 |
1 |
6 |
52 |
| NSW |
609 |
23 |
22 |
654 |
| NT |
66 |
2 |
1 |
69 |
| Qld |
412 |
24 |
5 |
441 |
| SA |
160 |
4 |
2 |
166 |
| Tas |
33 |
2 |
0 |
35 |
| Vic |
400 |
21 |
15 |
437 |
| WA |
135 |
2 |
5 |
142 |
| Australia |
1,860 |
79 |
56 |
1,995 |
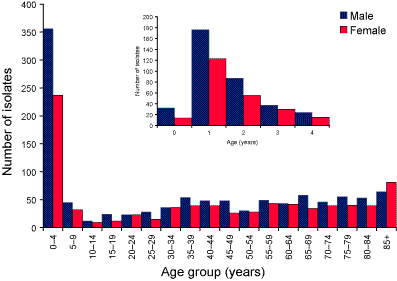
The number of isolates by the age and sex of the patient is shown in Figure 1. There were more isolates from males than females (male to female ratio 1.3:1), which was the same sex ratio as seen in the notification data. The largest number of isolates were from children aged 1 year (Figure 1).
Figure 1. Pneumococcal isolates, Australia, 2003 by age and sex
Top of page
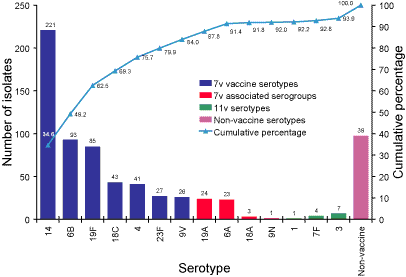
Serotypes responsible for invasive pneumococcal disease in Australian children less than five years of age and proportion represented in conjugate vaccines.
Six hundred and thirty-eight pneumococcal isolates from children less
than five years of age were serotyped. The serotype distribution proportion
of isolates from this age group represented in the 7vPCV and prototype 11vPCV
conjugate pneumococcal vaccines are illustrated in Figure 2. Eighty-four
percent of isolates were a serotype match for the 7vPCV vaccine and 92 per
cent of isolates were a serogroup match. The future 11vPCV vaccine (addition
of serotypes 1, 7F, 5 and 3) would add another 1.9 per cent of isolates belonging
to vaccine serotypes.
Figure 2. Serotypes responsible for invasive pneumococcal disease in children less than five years, Australia,
2003
Pneumococcal serotypes with reduced susceptibility to penicillin
and erythromycin in Australian children less than five years of age
Of the 638 isolates from children less than five years of age that were
serotyped, 622 also had penicillin susceptibility results recorded. Overall,
71/622 (11%) had reduced susceptibility to penicillin. Sixty of these (85%)
were serotypes and 70 (99%) were serogroups in the 7vPCV (Table 3).
Table 3. Serotypes of isolates with reduced susceptibility to penicillin in children aged less than five years, Australia, 2003 (N = 71)
| Serotype |
19F* |
9V* |
14* |
6B* |
23F* |
19A† |
6A† |
33F‡ |
Total |
| Number of isolates |
19 |
16 |
16 |
8 |
1 |
6 |
4 |
1 |
71/622 |
| Cumulative % |
27% |
49% |
72% |
83% |
85% |
93% |
99% |
100% |
11% |
Of the 638 isolates from children less than five years of age that were
serotyped, 567 also had susceptibility results for erythromycin recorded.
Overall, 138/567 (24%) were resistant to erythromycin. One hundred and thirty-four
of the 138 erythromycin resistant isolates were a serotype match for the
7vPCV and the remaining four isolates were a serogroup match (Table 4). The
majority (70%) of erythromycin resistant isolates in children less than five
years of age in Australia were
serotype 14.
Table 4. Serotypes with reduced susceptibility to erythromycin in children aged less than five years, Australia, 2003 (n=138)
| Serotype |
14* |
19F* |
6B* |
18C* |
23F* |
4* |
19A† |
6A† |
Total |
| Number of isolates |
97 |
17 |
16 |
1 |
2 |
1 |
1 |
3 |
138/567 |
| Cumulative % |
70% |
83% |
94% |
95% |
96% |
97% |
98% |
100% |
24% |
The rates of penicillin and erythromycin resistant pneumococci in children
aged less than five years of age by state and territory are shown in Table
5. The overall rate of penicillin resistance and erythromycin resistance
varied widely between states. There were no penicillin resistance isolates
identified in the Northern Territory or Tasmania and rates ranged from five per cent in South Australia to 14 per cent in New South Wales. Erythromycin resistance was found in all states ranging
from six per cent in the Northern Territory to 75 per
cent in Tasmania, although the
latter rate was based on only four isolates.
Top of page
Table 5. Penicillin and erythromycin resistance in children aged less than five years, Australia, 2003, by state and territory
| State |
ACT |
NSW |
NT |
Qld |
SA |
Tas |
Vic |
WA |
Australia |
| Penicillin |
| Proportion of isolates tested |
11/11 |
211/212 |
18/19 |
159/160 |
65/66 |
5/5 |
116/128 |
37/37 |
622/638 |
| Number (%) Penicillin reduced susceptibility |
1 (9%) |
30 (14%) |
0 (0%) |
22 (14%) |
3 (5%) |
0 (0%) |
12 (10%) |
3 (8%) |
71 (11%) |
| Erythromycin |
| Proportion of isolates tested |
11/11 |
201/212 |
18/19 |
158/160 |
63/66 |
4/5 |
75/128 |
37/37 |
567/638 |
| Number (%) Erythromycin resistant |
3 (27%) |
57 (28%) |
1 (6%) |
49 (31%) |
15 (24%) |
3 (75%) |
5 (7%) |
5 (14%) |
138 (24%) |
Differences in the prevalence of serotype 14—which accounted for 70 per
cent of erythromycin resistance—largely reflected differences in erythromycin
resistance rates between jurisdictions.
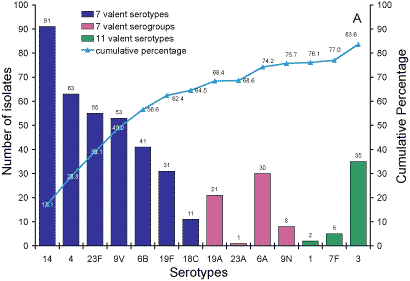
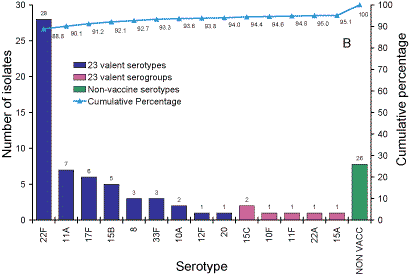
Serotypes responsible for IPD in Australian adults over 65
years of age.
Five hundred and thirty-five isolates from adults aged more than 65 years
were analysed. Sixty-five percent of isolates were 7vPCV serotypes and 76
per cent 7vPCV serogroups (Figure 3, Panel A). 84 per cent of serotypes were
in the 11vPCV conjugate vaccine and 94 per cent were serotypes in the 23vPPV
polysaccharide vaccine (Panel B).
Figure 3. Serotypes responsible for invasive pneumococcal disease in adults aged more than 65
years, Australia, 2003. Panel A serotypes in 7- and 11vPCV vaccines; Panel B Serotypes in the 23vPPV vaccine
The likely impact (via herd immunity) on incidence of IPD in the adult
population over 65 years of age following the introduction of the universal
childhood immunisation program with the 7vPCV vaccine in Australia was assessed
based on the reductions seen in the USA (14). Based on the serotype distribution
in the elderly in 2003 it was estimated that 110 cases of IPD would be prevented
in adults over 65 years of age as a result of herd immunity associated with
universal 7vPCV vaccination in Australian children (Table 6).
Top of page
Table 6. Predicted number of cases of invasive pneumococcal disease in adults aged more than 65 years that could be prevented as a result of introduction of 7vPCV vaccine in children in Australia
| Serotype |
Number of isolates |
Per cent change post vaccine* |
Number of cases prevented |
14 |
91 |
-36% |
33 |
4 |
63 |
-26% |
16 |
23F |
55 |
-31% |
17 |
9V |
53 |
-36% |
19 |
6B |
41 |
-16% |
7 |
19F |
31 |
-4% |
1 |
18C |
11 |
-31% |
3 |
19A |
21 |
-22% |
5 |
23A |
1 |
-22% |
<1 |
6A |
30 |
-22% |
7 |
9N |
8 |
-22% |
2 |
Overall |
535 |
-20% |
110 |
Further, based on the serotype prevalence in the 20 to 39 year age group
(n=263), approximately 54 cases in this age group should be prevented by
the 7vPCV vaccination of Australian children (data not shown).
Differences in penicillin and erythromycin resistance rates
by age and serotype
The proportion of penicillin resistant pneumococci in Australians over
65 years of age was significantly higher (17%) than the proportion in children
less than five years of age (11%) Table 7.
Table 7. Proportions of penicillin resistant pneumococcal isolates by age group and serotype, Australia,
2003
|
Serotype |
Children aged less than five years |
Adults aged over 65 years |
Significance of difference |
| Proportion |
Per cent |
Proportion |
Per cent |
| Total isolates tested |
622/638 |
97 |
514/535 |
96 |
NS |
| Resistant serotypes |
71/622 |
11 |
85/514 |
17 |
p<0.05 |
| 7v vaccine serotypes* |
60/71 |
84 |
76/85 |
89 |
NS |
| Serotype 19F |
19/85 |
22 |
15/31 |
48 |
p<0.05 |
| Serotype 14 |
16/221 |
7 |
18/91 |
19 |
p<0.005 |
| Serotype 6B |
8/93 |
9 |
8/41 |
19 |
NS |
| Serotype 9V |
16/26 |
61 |
31/53 |
58 |
NS |
| Serotype 23F |
1/27 |
4 |
3/55 |
5 |
NS |
The converse was true for erythromycin resistance with a significantly
higher proportion of resistant isolates (24%) in children less than five
years of age compared to adults over 65 years of age (15%, Table 8). This
difference was contributed to by the larger proportion of erythromycin-resistant
serotype 14 isolates in children.
Top of page
Table 8. Proportion of erythromycin resistant pneumococcal isolates by age group and serotype, Australia, 2003
| Serotype |
Children aged less than five years |
Adults aged over 65 years |
Significance of difference |
| Proportion |
Per cent |
Proportion |
Per cent |
| Total isolates tested |
567/638 |
89 |
474/535 |
89 |
NS |
| Resistant serotypes |
138/567 |
24 |
69/474 |
15 |
p<0.001 |
| 7v vaccine serotypes* |
135/138 |
98 |
58/69 |
84 |
p<0.001 |
| Serotype 19F |
17/85 |
20 |
11/31 |
35 |
NS |
| Serotype 14 |
97/221 |
44 |
28/91 |
31 |
p<0.05 |
| Serotype 6B |
16/93 |
17 |
6/41 |
15 |
NS |
| Serotype 9V |
0/26 |
0 |
1/53 |
2 |
NS |
| Serotype 23F |
2/27 |
7 |
7/55 |
13 |
NS |
Differences in serotype distribution and penicillin resistance of CSF
isolates in patients less than five years of age compared those over five
years of age
Pneumococcal isolates from the CSF of patients over five years of age
were more likely to be resistant to penicillin than those from patients less
than five years of age, but this difference did not reach statistical significance.
Serotypes 6B and 14 accounted for a significantly higher proportion of pneumococcal
isolates from the CSF of patients over five years of age than those less
than five years of age. Serotype 19F was more common in patient aged five
years or more but the difference in proportion was not significant (Table
9).
Table 9. Proportions of penicillin resistant isolates and common serotypes isolated from cerebrospinal fluid, by age group, Australia, 2003
Serotype in CSF |
Cases aged less than 5 years(n=34) |
Cases aged 5 years and above (n=45) |
Significance of difference |
| Proportion |
Percentage |
Proportion |
Percentage |
| Penicillin resistant isolates |
3/32 |
9 |
11/41 |
27 |
NS |
| Serotype 19F |
1/34 |
3 |
9/45 |
20 |
NS |
| Serotype 6B |
9/34 |
26 |
2/45 |
4 |
p<0.05 |
| Serotype 14 |
11/34 |
32 |
3/45 |
6 |
p<0.01 |
| Serotype 9V |
2/34 |
6 |
5/45 |
11 |
NS |
Top of page
Discussion
The impact of a universal 7vPCV program for young children on invasive
pneumococcal disease has now been clearly demonstrated in the United States
of America (USA).14 The
vaccine program has benefited not only young children but it has also their
parent’s age group (20 to 39 years) and the elderly in whom the rates of
IPD have also decreased. Recently the National Health and Medical Research
Council has recommended 7vPCV for all children in Australia as part of their
primary immunisation series18 and the Australian
government has undertaken to fund this initiative from January 2005. Reliable
baseline data on serotype prevalence in Australian children is essential
to measure the impact of this new vaccine program. This study has examined
serotype distribution and antimicrobial resistance in more than 90 per cent
of the notified cases of IPD in Australia in
2003. These data allow us to predict the likely benefits which will be seen
as a result of this new vaccine initiative.
A large proportion of young Australian children with IPD in 2003 were
infected with pneumococcal serotypes (84%) or serogroups (92%) in the 7vPCV
vaccine. While still not conclusive, some cross-protection for serogroups
contained in the vaccine have been reported.14
It is therefore reasonable to predict that Australia will see a significant
decline in IPD in young children in the coming years when the new 7vPCV vaccination
program is fully implemented. This could be of the order seen in the USA,
where IPD declined by 69 per cent in the under 2-year olds in the first two
years of a universal childhood vaccination program in this age group.14
The serotype distribution of penicillin resistant pneumococcal strains
in young Australian children showed that 85 per cent of penicillin resistant
isolates were a serotype and 99 per cent were a serogroup in 7vPCV. There
is evidence from the USA that the rate of IPD due to penicillin resistant
strains can be expected to fall by as much as 35 per cent with introduction
of the 7vPCV.14
There are significant regional differences in penicillin and erythromycin
resistance in Australia. Victoria
in particular appears to have relatively low rates of erythromycin resistance
and a low prevalence of serotype 14 which is frequently erythromycin resistant.
A recent study by the NSW Pneumococcal Reference Laboratory has identified
the predominant penicillin susceptible, erythromycin resistant clone of serotype
14 in NSW to be multi-locus sequence type (MLST) 9 (M. Watson, unpublished
observations). The molecular mechanism of resistance to erythromycin in these
MLST9 strains in New South Wales
appears to be due to the macrolide efflux gene (mef)
which does not confer cross resistance to the lincosamides such as clindamycin
(M. Watson, unpublished observations). Serotype 14 erythromycin resistant
isolates accounts for over 80 per cent of macrolide resistant isolates in
children in Australia.
By contrast the Northern Territory has a very low prevalence
of macrolide resistance in children probably due to the virtual absence of
serotype 14 following the introduction of the 7vPCV vaccination program in
2001 for all indigenous children in the NT and non-Indigenous children in Central
Australia. Variations in rates of antibiotic prescribing and consumption
would also explain variation in the prevalence of antimicrobial resistance
across Australia and between age groups.
In the first two years of the childhood 7vPCV vaccine program in the
United States there were declines in IPD disease rates in adults (32% in
the 20 to 39 year age group and 18% in the aged 65 years or older).14
Extrapolating the reductions in the prevalence of 7vPCV vaccine serotypes
seen in the elderly population in the USA,14 we
predict that 110 (20%) episodes of IPD in adults over 65 years of age and
54 (20%) cases in the 20–39 year age group in Australia could be prevented
as a result of the paediatric immunisation program. These calculations, based
as they are on incomplete data, may under-estimate the eventual impact of
universal 7vPCV vaccination
There may also be additional benefits of 7vPCV vaccination by reductions
in the prevalence of antibiotic resistant isolates in the elderly through
improved herd immunity. However the prevalence of resistance varies significantly
between children and adults. This appears to be associated with variations
in the prevalence of penicillin and erythromycin resistant clones rather
than existence of genetically distinct molecular clones in the two populations
(M. Watson unpublished observations). The relatively higher prevalence of
penicillin resistant serotypes in the elderly suggests that a reservoir of
penicillin resistance exists in the elderly population in Australia with
significant selective pressure towards acquisition of penicillin resistant
strains of serotype 19F, 14 and 6B occurring in this age group. It is likely
that immunising children with the 7vPCV will reduce the incidence of penicillin
resistant serotypes in the elderly since children would be less likely to
pass on this serotype to their ‘grandparents’. Reductions in IPD caused by
serotype 19F and 6B have not been clearly demonstrated in the USA at this time,14
however a reduction of serotype 14 IPD in the elderly has been
observed. In 2003, serotype 19F in the elderly was a cause of meningitis
in the older age group and three of the four penicillin resistant serotypes
isolated in CSF from people over 65 years of age in Australia.
The 23vPPV polysaccharide pneumococcal vaccine continues to provide good
serotype coverage for adults, which supports the recent government decision
to fully fund this vaccine for those at risk in Australia.
The continued laboratory surveillance of IPD is a vital component of
the pneumococcal vaccine strategy for Australia. The funding of this surveillance has
assisted a national approach to surveillance and reporting of this important
reference laboratory work. Committed funding for serotype and uniform antibiotic
susceptibility testing would assure appropriate monitoring of the impact
of the new universal childhood 7vPCV program. Our surveillance to date suggests
that introduction of the 7vPCV for all children and the 23vPPV vaccine for
the 65 years and over in Australia in 2005 is likely to lead to major
benefits for both children and the elderly.
Top of page
Acknowledgements
List of Contributors to Pneumococcal Laboratory Surveillance
Australian Capital Territory
The Canberra Hospital
Prof Peter Collignon and Ms Susan Bradbury
New South Wales
The Children’s Hospital, Westmead
Ms Gail Stewart, Mrs Maggie Brett, Mrs Shirley Warren, Mr Mitchell Brown
Central Coast Pathology
Dr Deo Dewitt, Mr Bruce Beaman
Concord Hospital
Dr Tom Gottlieb, Ms Candice Wolfson
Davies Campbell and De Lambert
Dr De Lambert, Mr Steve Hodges
Douglass Hanley Moir
Dr Ian Chambers, Mr Richard Jones
Hunter Area Pathology
Dr John Ferguson, Mr Chris Ashurst-Smith
CIDM, ICPMR
Prof Lyn Gilbert, Mr David Smith
Laverty Pathology
Dr
Len Moaven, Mr David Rankin
THE Pathology
Dr Val Ackerman, Mr Fuad
Teppo
Nepean Hospital
Dr James
Branley, Mr David Rose
PaLMS
Robert Prichard, Dr Clarence Fernandez
Royal Prince Alfred Hospital
Prof
Richard Benn, Ms Barbara Yan
Top of page
SEALS
Prof John Tapsall, Ms Sue Mahrer
St George
Dr
Peter Taylor, Ms Kerry Varettas
ST Vincents Hospital
Dr Jock Harkness,
Ms Robyn Timmins
SWAPS
Dr
Iain Gosbell, Mr Steven Neville
Sydney Adventist Hospital
Dr
Ross Bradbury, Dr Ross Grant
Wollongong Hospital
Dr
Peter Newton, Mr Nelson Dennis
Special thanks to Ms Robin Gilmour from NSW Health
Northern Territory
Royal Darwin Hospital, Dept of Microbiology
Private laboratories in the NT
Alice Springs Hospital
Dept of Microbiology
Katherine Hospital
Dept of Microbiology
Public Health Unit
Queensland
Queensland Health Pathology
Laboratories and the Microbiology Discipline Working Party
Private Pathology Laboratories throughout Queensland
Tropical Public Health Unit, Cairns
Dr Jeffrey Hanna
Communicable Diseases Unit, Brisbane
Dr Margaret Young, Dr Robyn Pugh
South Australia
Women’s and Children’s Hospital, Adelaide
The Gribbles Group, SA
South Path Microbiology and Infectious Diseases
Clinipath Laboratories
Institute for Medical and Veterinary Science laboratories, SA
Top of page
Tasmania
Robert Peterson, Royal Hobart Hospital
(Department of Microbiology)
Mr Robert Peterson
Launceston General Hospital
(Northern Tasmanian Pathology Service)
Mr Peter Dadson
Hobart Pathology
Mr Gary Fenton
North West Pathology
Ms Tara
Carswell
Special thanks to Mr David Coleman from CDPU, Department of Health and
Human Services
Victoria
MDU PHL is grateful to the following labs who have
been identified as having contributed isolates to the reported
data-set:
Box Hill Hospital Pathology Service
Royal Childrens Hospital
(Parkville) Pathology Service
Dorevitch Pathology Mayne Health (Heidelberg)
Gippsland Pathology Service Sale
(& Traralgon)
Alfred Hospital Pathology Service
Monash Medical Centre (Clayton) Pathology Service
Austin Hospital Pathology Service
Bendigo Health Pathology Service
Goulburn Valley Health (Shepparton) Pathology Service
Northern Hospital (Epping) Pathology Service
St John of God Health Care Ballarat
Pathology Service
Geelong Hospital Pathology Service (Pathcare)
South West Healthcare (Warnambool) Pathology Service
Saint Frances Xavier Cabrini Hospital Pathology Service
Royal Melbourne Hospital
(Parkville) Pathology Service
St Vincents Hospital (Melbourne) Ltd Pathology
Service
Ballarat Health Services (Base campus) Pathology Service
Forensicare - Victorian Institute of Forensic Medicine
Wimmera Base Hospital (Horsham) Pathology Service
Gribbles Pathology (Melbourne)
Echuca Hospital Pathology Service
Mildura Base Hospital Pathology Service
Melbourne Pathology
St John of God Health Care Mildura
Pathology Service
From MDU PHL, Ms Janet Strachan contributed to testing, Dr Mark Veitch
and Ms Sally Bodenham to data management.
Western Australia
We would like to acknowledge the Vaccine Impact Surveillance Network
which is funded by the Meningitis Centre of Western Australia and The Telethon
Institute for Child Health Research
Princess Margaret and King Edward Memorial Hospitals Dept Microbiology
Fremantle Hospital
Dept Microbiology
PathCentre
Royal Perth Hospital Dept Microbiology
St John of God Pathology Dept
Microbiology
Western Diagnostic Pathology Dept Microbiology
Clinipath Dept of Microbiology
Special Thanks to Ms Carolien Giele from CDCP, Health Dept of Western Australia
Top of page
References
1. Kertesz DA, Di Fabio
JL, de Cunto Brandileone MC, Castaneda E, Echaniz-Aviles G, Heitmann I, et
al. Invasive Streptococcus Pneumoniae Infection in Latin American
Children: Results of the Pan American Health Organization Surveillance Study.
Clin Infect Dis
1998 Jun;26:1355–1361.
2. Jette LP, Lamothe F. Surveillance
of Invasive Streptococcus Pneumoniae Infection in Quebec, Canada,
From 1984 to 1986: Serotype Distribution, Antimicrobial Susceptibility, and
Clinical Characteristics. J Clin Microbiol 1989;27:1–5.
3. Nielsen SV, Henrichsen
J. Capsular Types of Streptococcus Pneumoniae Isolated From Blood and CSF During 1982–1987. Clin
Infect Dis 1992;15:794–798.
4. Voss L, Lennon D, Okesene-Gafa
K, Ameratunga S, Martin D. Invasive Pneumococcal Disease in a Pediatric Population,
Auckland, New Zealand. Pediatr Infect
Dis J 1994;13:873–878.
5. McIntyre PB, Gilmour
RE, Gilbert GL, Kakakios AM, Mellis CM. Epidemiology of Invasive Pneumococcal
Disease in Urban New South Wales, 1997–1999. Med
J Aust 2000;173 Suppl:S22–S26.
6. Krause VL, Reid SJ, Merianos
A. Invasive Pneumococcal Disease in the Northern Territory
of Australia,
1994–1998. Erratum Appears in MJA
2001;174:309. Med J Aust
2000;173 Suppl:S27–S31.
7. Hogg GG, Strachan JE, Lester
RA. Invasive Pneumococcal Disease in the Population of Victoria. Med
J Aust 2000;173 Suppl:S32–S35.
8. VISN. Are Current Recommendations
for Pneumococcal Vaccination Appropriate for Western Australia? The Vaccine Impact Surveillance
Network—Invasive Pneumococcal Study Group. Med J Aust 2000;173
Suppl:S36–S40.
9. Andresen DN, Collignon
PJ. Invasive pneumococcal disease in the Australian
Capital Territory and Queanbeyan region: do high infant rates reflect more
disease or better detection? J Paediatr
Child Health 2004;40:184–188.
10. Turnidge JD, Bell JM, Collignon PJ. Rapidly Emerging Antimicrobial
Resistances in Streptococcus Pneumoniae in Australia. Pneumococcal
Study Group. Med J Aust
1999;170:152–155.
11. Watson M, Bayley K, Bell JM,
Gilbert GL, Hogg G, Keil AD, et al. Laboratory surveillance
of invasive pneumococcal disease in Australia in 2001 to 2002 – implications for
vaccine serotype coverage. Comm Dis Intell
2003;27:478–487.
12. Roche P, Krause V, Bartlett M,
Coleman D, Cook H, Counahan M, et al. Invasive pneumococcal disease in Australia, 2003. Comm Dis Intell
2004;28:441–454
13. Lund E, Henrichsen J. Laboratory diagnosis,
serology and epidemiology of Streptococcus pneumoniae.
In Bergan T, Norris R. eds Methods in Microbiology,
Volume 12 Acadamic Press, London, 1978, pp241–262.
14. Whitney CG, Farley MM, Hadler J, Harrison
LH, Bennett NM, Lynfield R, et al. Decline in Invasive
Pneumococcal Disease After the Introduction of Protein-Polysaccharide
Conjugate Vaccine. N Engl J Med
2003;348:1737–1746.
15. National Committee for Clinical
Laboratory Standards. Preformance Standards for Antimicrobial disk susceptibility
tests;approved standards. 8th edition, NCCLS document, m2–A8, Villanova,
2003.
16. Bell SM, Gatus BJ, Pham JN, Rafferty
DL. Antibiotic susceptibility testing by the CDS method. A
manual for medical and veterinary laboratories. The Antibiotic Reference
Laboratory, South Eastern Area Laboratory Services, 2002.
17. E-test Technical Manual. Technical
Guide 5C:Susceptibility testing of pneumococci,
2000.
18. National Health and Medical Research
Council. The Australian Immunisation Handbook 8th Edition. National
Health and Medical Research Council. 2003 pp 143–152.
Top of page
Author affiliations
1. The NSW Pneumococcal Reference Laboratory, Department of Microbiology, The Children’s Hospital at Westmead, Westmead, New South Wales
2. Surveillance Section, Australian Government Department of Health and Ageing, Canberra, Australian Capital
Territory
3. The Department of Microbiology and Infectious Diseases, Adelaide Women’s and
Children’s Hospital, Adelaide, South Australia
4. Infectious Diseases Unit and Microbiology Department, The Canberra Hospital, Garran, Australian
Capital Territory
5. Centre for Infectious Diseases and Microbiology, Institute of Clinical Pathology
and Medical Research, Westmead Hospital, Westmead, New South Wales
6. Microbiological Diagnostic Unit, Public Health Laboratory, Microbiology and Immunology Department, The University
of Melbourne, Melbourne, Victoria
7. The Department of Microbiology, Women’s and Children’s Health Service, Western Australia.
8. Centre for Disease Control, Department of Health and Community Services, Casuarina, Northern
Territory
9. Pneumococcal Reference Laboratory, Queensland Health Scientific Services, Queensland
Corresponding author: Dr Michael Watson, Clinical Microbiologist and
Infectious Disease Physician, St John of God Pathology, Hollywood Hospital, Monash
Avenue, Nedlands, Western Australia 6009. Telephone: +61 8 9284 8181. Facsimile: +61 8 9386 9852. Email: michael.watson@sjog.org.au
This article was published in Communicable Diseases Intelligence Vol 28 No 4, December 2004
