Streptococcus pneumoniae is a major cause of morbidity and mortality in young children throughout the world, causing both invasive (meningitis, bacteraemia) and non-invasive (pneumonia, acute otitis media, sinusitis) infections. Over the past few decades, the global emergence of antibiotic-resistant pneumococcal strains has complicated disease management. Thus, healthcare practitioners have begun to place more emphasis on the judicious use of antibiotics and prevention of disease through routine immunisation. Researchers have developed several pneumococcal conjugate vaccines, which due to their technology, are effective in infants and young children. Currently, one 7-valent pneumococcal conjugate vaccine (PNCRM7; Prevenar®, Wyeth) is available in various parts of the world and has demonstrated excellent efficacy against vaccine-type invasive disease and efficacy against pneumonia and otitis media caused by the serotypes included in the vaccine. Furthermore, there is evidence suggesting that the use of these conjugate vaccines will reduce the need for antibiotics and the subsequent spread of antibiotic-resistant pneumococci. Ultimately, when routine pneumococcal conjugate vaccination of infants and young children is accompanied by supportive education and active disease surveillance as well as judicious use of antibiotics, there should be a favourable impact on pneumococcal disease incidence in and beyond the vaccinated population. Commun Dis Intell 2003;27 Suppl:S134-S142.
Top of page
Introduction
Streptococcus pneumoniae is a major cause of morbidity and mortality in young children throughout the world.1 The leading cause of bacterial meningitis in infants and children,2S. pneumoniae also causes other invasive infections, such as bacteraemia and bacteraemic pneumonia. Non-invasive infections commonly caused by S. pneumoniae include non-bacteraemic pneumonia, acute otitis media (AOM) and sinusitis.1
The annual incidence of invasive pneumococcal disease in Australia ranges from 31.7 to 2,053 cases per 100,000 population, depending on the geographic region, age, and ethnic background of the population studied.3,4,5,6,7 As shown in Figure 1, the incidence of disease is high among children younger than 2 years, with the highest incidence seen in indigenous children.
Figure 1. Annual incidence of invasive pneumococcal disease in various regions of Australia3,4,5,6,7

Over the past few decades, the global emergence of antibiotic-resistant pneumococcal strains has complicated disease management. Thus, healthcare practitioners have begun to place more emphasis on the judicious use of antibiotics and prevention of disease through routine immunisation.8,9 This article reviews the increasing incidence of pneumococcal antibiotic resistance and the potential role of pneumococcal conjugate vaccines in reducing antibiotic use and the spread of antibiotic-resistant pneumococcal strains.
Top of page
Microbiology, transmission, and carriage of pneumococci
Microbiology
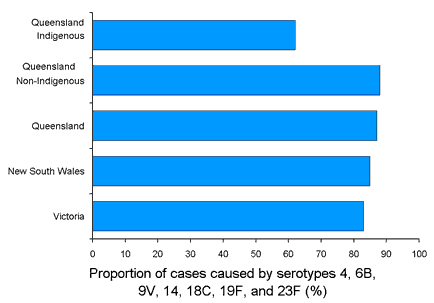
Pneumococci are gram-positive, lancet-shaped bacteria that occur in chains (streptococci) or in pairs (diplococci) and are typically surrounded by a large complex polysaccharide capsule. Based on differences in the composition of the polysaccharide capsule, there are approximately 90 known serotypes of S. pneumoniae. The prevalence of different serotypes varies by age, geographic location, and type of disease.10 In the Australian population, 7 serotypes (4, 6B, 9V, 14, 18C, 19F, 23F) cause between 62 per cent and 88 per cent of invasive pneumococcal disease among children younger than 5 years (Figure 2).3,5,6,11 Interestingly, the proportion of invasive pneumococcal disease caused by these serotypes is somewhat lower among young children of indigenous descent.5,12
Figure 2. Proportion of invasive pneumococcal disease cases attributed to serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F, by region3,5,6,11
Nasopharyngeal carriage and transmission
Asymptomatic nasopharyngeal carriage of pneumococci is widely prevalent among young children (ranging from 28% to 86% throughout the United States of America and Europe) and is often the first step in disease transmission.13,14,15,16 Consequently, young children play an important role in the transmission of pneumococcal disease in the community because of their high carriage rate and the ease with which they can transmit the disease through expulsion of respiratory droplets. Consistent with the findings from other countries, studies in southern Australia among children younger than 5 years of age have found pneumococcal nasopharyngeal carriage rates ranging between 37 per cent and 52 per cent.17 Of particular note, half of these isolates were resistant to antibiotic treatment.17
Top of page
Pneumococcal antibiotic resistance
Historically, penicillin has been the agent of choice for treatment of pneumococcal disease; however, the widespread use and overuse of penicillin has resulted in the development of penicillin-non-susceptible pneumococcal strains and necessitated changes in treatment.8,9,18,19,20 Penicillin non-susceptibility-the degree of resistance to treatment-is classified according to minimum inhibitory concentrations (MICs), defined as the minimum concentration of a particular antibiotic needed to stop pneumococcal growth in vitro (Table 1).21,22
Table 1. Definition of penicillin resistance21,22
Level of resistance* |
Minimum inhibitory concentration |
| Susceptible |
≤ 0.06 µ g/mL |
| Intermediate susceptibility |
0.1–1 µ g/mL |
| Resistant |
≥2 µ g/mL |
Rates of pneumococcal non-susceptibility vary geographically and are rising throughout the world. For example, penicillin non-susceptibility in Spain increased from 6 per cent in 1979 to 44.3 per cent in 1989. By 1999, approximately 60 per cent of all pneumococcal isolates were penicillin non-susceptible.23,24 Similarly, in the United States of America (USA), an 11-fold increase in the rate of penicillin non-susceptibility was observed between the years 1986 (3.8%) and 1997 (43.8%).25 The proportion of penicillin-non-susceptible pneumococci has increased in south-eastern Australia as well. In 1990, approximately 2 per cent of isolates were intermediately resistant to penicillin; by 2000, approximately 10 per cent of isolates from blood and cerebrospinal fluid (CSF) cultures and just over 35 per cent of isolates from sites other than blood and CSF exhibited non-susceptibility to penicillin.19 Further evidence of the dramatic increase in antibiotic resistance is provided by a 1997 Australian-wide surveillance study showing that approximately 25 per cent of the 1,020 isolated strains were non-susceptible to penicillin (16.8% were intermediately resistant and 8.6% were resistant).26 Rates of resistance to other drugs were also relatively high, with 15.6 per cent of strains resistant to erythromycin, 15.7 per cent resistant to tetracycline, 21.4 per cent resistant to cefaclor, 33.4 per cent resistant to cotrimoxazole, and 3.1 per cent each resistant to amoxicillin-clavulanate and ceftriaxone.26
Top of page
Not surprisingly, the problem of multidrug-resistant strains has been growing at an alarming rate worldwide.9,19,26 For example, a 1997 USA surveillance study reported that 36.7 per cent of penicillin-intermediate and 65.6 per cent of penicillin-resistant isolates were also resistant to macrolide antibiotics.27 With respect to Australia, Gratten and colleagues identified 27 cases of multidrug-resistant S. pneumoniae in Queensland between 1995 and 1996.28 All 27 isolates demonstrated resistance to cotrimoxazole, 19 strains (70%) were resistant to chloramphenicol, 25 strains (93%) were resistant to erythromycin, and 25 strains (93%) were resistant to tetracycline. Penicillin-non-susceptible strains were recovered from 18 of the 27 multidrug-resistant cases (66.7%). Furthermore, 14 penicillin-resistant isolates were also resistant to ceftriaxone. The serotype distribution of these multidrug-resistant pneumococci included serotype 19F (15 isolates), serotype 14 (6 isolates), serotype 23F (4 isolates), serotype 6A (1 isolate), and serotype 19A (1 isolate).
The spread of antibiotic-resistant pneumococci has been associated with out-of-home childcare attendance and previous antibiotic use.29,30,31 Day care attendance increases the risk of resistant pneumococcal disease due to frequent contact with other children, exposure to a greater number of serotypes, and difficulty in maintaining hygienic conditions.30.31 Furthermore, Levine and colleagues reported that children in day care are more likely to have had a recent ear infection and more likely to have one recent course of antibiotics.30
Taken together, the dramatic increase in the prevalence of antibiotic-resistant pneumococci and the persistently high morbidity and mortality associated with pneumococcal infections has shifted the focus of disease management from antibiotic therapy to the prevention of infection through immunisation.2,8,9
Top of page
Role of vaccination in reducing antibiotic resistance
Although pneumococcal polysaccharide vaccines have been available since the 1980s, they are ineffective in children younger than 2 years because they produce a T-cell-independent immune response.32 To overcome this problem, researchers have developed several pneumococcal conjugate vaccines based on the same principles used to develop the highly successful Haemophilus influenzae type b vaccine. Table 2shows the serotypes and carriers used in the various pneumococcal conjugate vaccines. The only pneumococcal conjugate vaccine available to date is a 7-valent vaccine (serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F) conjugated to a non-toxic diphtheria variant (CRM197) (PNCRM7; Prevenar®; Wyeth).
Table 2. Composition of pneumococcal conjugate vaccines
Vaccine |
Protein carrier |
Serotypes |
Manufacturer |
| PNCRM7 |
Non-toxic variant (CRM197) |
4, 6B, 9V, 14, 18C, 19F, 23F |
Wyeth |
| PNCRM9 |
CRM197 |
1, 4, 5, 6B, 9V, 14, 18C, 19F, 23F |
Wyeth |
| PNCOMP7 |
Outer membrane of Neisseria meningitidis group
B |
4, 6B, 9V, 14, 18C, 19F, 23F |
Merck and Co. |
| PNC–D/T |
Diphtheria and tetanus toxoids |
1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, 23F |
Aventis-Pasteur |
| PNC–protein D |
Haemophilus influenzae protein D |
1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, 23F |
GlaxoSmithKline |
PNCRM7 has demonstrated excellent efficacy against vaccine-type invasive disease and moderate efficacy against vaccine-type AOM. In the first of two safety and efficacy trials, the Northern California Kaiser Permanente (NCKP) study, subjects were randomly assigned to immunisation with PNCRM7 or the meningococcal group C conjugate vaccine (control).2 Efficacy of PNCRM7 against vaccine-serotype-specific invasive disease was 97.4 per cent in the per-protocol analysis (received ³ 3 doses) and 93.9 per cent in the intent-to-treat analysis (received ³ 1 dose). In addition, there was a 6.4 per cent decrease in otitis media episodes, a 9.1 per cent reduction in frequent AOM episodes, and a 20.3 per cent reduction in tympanostomy tube insertions.
Postlicensure surveillance from this trial has shown that the incidence of invasive disease among all children in the NCKP healthcare system (vaccinated and unvaccinated) after licensure and routine use of the vaccine was reduced in children younger than 1 year, 2 years, and 5 years by 87.3 per cent, 58.1 per cent, and 62.4 per cent, respectively, thus suggesting that the benefits of pneumococcal immunisation may extend to the non-vaccinated population as well (i.e., indirect or herd immunity).33
Top of page
The second PNCRM7 study used a vaccination schedule similar to that of the NCKP study; 1,662 subjects were randomly assigned to immunisation with PNCRM7 or the hepatitis B vaccine (control).34 Similar to the previous study, vaccination with PNCRM7 reduced the incidence of all AOM episodes by 6 per cent, while the incidence of culture-confirmed AOM episodes and vaccine-serotype episodes decreased by 34 per cent and 57 per cent, respectively. However, an increase of 33 per cent was observed for episodes caused by non-vaccine serotypes.
The potential effects of the pneumococcal conjugate vaccines on antibiotic resistance have been examined in 876 children with pneumococcal AOM in southern Israel.35 Analysis of middle ear fluid isolates showed that 68 per cent were resistant to one or more antibiotic, 61 per cent were resistant to penicillin, and 13 per cent were resistant to three or more antibiotic classes. Taken together with the serotype composition of such isolates (primarily 6B, 9V, 14, 19F, and 23F), it is likely that pneumococcal conjugate immunisation will have a significant impact on the spread of antibiotic resistance, as these five serotypes are included in all of the pneumococcal conjugate vaccines.
Evidence suggesting that the benefits of pneumococcal vaccination extend beyond the prevention of disease to reducing the need for antibiotic treatment has been demonstrated. In the NCKP trial described previously, it was found that PNCRM7 reduced antibiotic use by 5.3 per cent.36 In another study conducted in children attending day care centres in Israel, researchers compared respiratory morbidity and antibiotic use among children receiving a 9-valent pneumococcal conjugate vaccine (PNCRM9) with that of children immunised with a meningococcal C conjugate (control) vaccine.37 Overall reductions were seen in the incidence of upper respiratory infections (15% decrease), lower respiratory infections (16% decrease), and AOM (17% decrease). In addition, the overall number of days of antibiotic use was significantly reduced (by 17%, p£ 0.005). When analysed by site of infection, antibiotic use in children with upper respiratory infections, lower respiratory infections, and AOM decreased by 10 per cent, 47 per cent, and 20 per cent, respectively (p£ 0.005 for all comparisons).37
Finally, preliminary data from the United States of America (presented at the American Society for Microbiology's Third International Symposium on Pneumococci and Pneumococcal Diseases in Anchorage, Alaska in May of 2002) suggest that the universal immunisation of infants and toddlers has dramatically reduced the incidence of antibiotic-resistant pneumococcal disease among individuals of all ages.
Top of page
Recommendations for use of PNCRM7 in Australia
PNCRM7 is indicated for the active immunisation of children 6 weeks to 9 years of age against invasive disease, pneumonia, and otitis media caused by those pneumococcal serotypes included in the vaccine (4, 6B, 9V, 14, 18C, 19F, and 23F). At this time, the Australian Advisory Group on Immunisation (ATAGI) recommends free (federally funded) vaccination with PNCRM7 to those children at greatest risk for pneumococcal disease.38 All eligible children (Table 3) should receive a 3-dose series, given at 2, 4, and 6 months of age. No booster dose is necessary for the majority of children, with 2 important exceptions: children with impaired immunity should receive a fourth booster dose, and certain Aboriginal and Torres Strait Islander children should receive a booster dose of the 23-valent polysaccharide vaccine (Pneumovax 23®, Merck and Co., West Point, PA). The recommended 'catch-up' schedule for eligible children older than 2 months of age is provided in Table 4.38 Finally, ATAGI is also currently considering inclusion of a pneumococcal conjugant vaccine as part of the routine, free immunisation schedule for all children, regardless of risk group.
Table 3. Children eligible for free vaccination with PNCRM738
Group |
Age limits |
| All Aboriginal and Torres Strait Islander children |
< 24 months |
| Non-Aboriginal children in Central Australia |
< 24 months |
| Aboriginal children in Central Australia or any region
with similarly high incidence of pneumococcal infections |
24–59 months |
| Children with medical risk factors for pneumococcal infection* |
< 5 years |
Table 4. Recommended catch-up PNCRM7 immunisation schedule38
| |
Age at first dose (months) |
Primary schedule (PNCRM7) |
Booster |
| Aboriginal and Torres Strait Islander children
in the Northern Territory, the desert and tropical regions of Western Australia
and Queensland, and the desert regions of New South Wales and southern
Australia |
3–6
7–17 |
3 doses*
2 doses* |
23V PS at 18–24 months |
18–24 |
1 dose |
23V PS 2 months later |
| Aboriginal children in Central Australia (and other regions
of similarly high pneumococcal disease incidence) |
24–59 |
1 dose |
23V PS 2 months later |
| Aboriginal and Torres Strait Islander children in all
other regions, non-Aboriginal children in Central Australia only, and children
with anatomical abnormalities |
3–6
7–23 |
3 doses*
2 doses* |
None
None |
| Children with impaired immunity |
3–6
7–11 |
3 doses*
2 doses* |
PNCRM7 at 12 months |
12–59 |
2 doses* |
None |
Top of page
Potential impact in Australia with widespread use of pneumococcal conjugate vaccines
Widespread use of PNCRM7 should lead to a decreased incidence of pneumococcal disease, indirectly reducing antibiotic use and the spread of antibiotic resistance. Epidemiology studies indicate that the seven serotypes included in PNCRM7 are responsible for 62-88 per cent of cases of invasive pneumococcal disease,3,5,6,11 suggesting that the majority of pneumococcal infections in children could be prevented by vaccination with PNCRM7. Although vaccine coverage would be expected to be lower among indigenous children (62%), the high incidence of disease in this group suggests that the vaccine will still be of substantial value.
In addition to the disease prevention effects in vaccinated individuals, immunisation of infants and young children with pneumococcal conjugate vaccines may indirectly extend disease prevention to a larger population (i.e., indirect or herd immunity), as observed in Northern California.
Top of page
Conclusions
To realise the full potential of pneumococcal conjugate vaccines, the implementation of vaccination programs must be accompanied by the education of medical practitioners and the public on the appropriate use of antibiotics. Moreover, continued disease surveillance is crucial for understanding whether replacement carriage or disease with non-vaccine serotypes occurs. To date, clinical trials have shown that immunisation with pneumococcal conjugate vaccines has resulted in decreased carriage of vaccine-type pneumococci and increased carriage of non-vaccine-type pneumococci;39,40,41,42 however, some replacement disease has only been observed for AOM.34 No replacement disease has been observed for invasive pneumococcal disease.33
It also remains to be determined if non-vaccine serotypes will begin to develop antibiotic resistance. Ultimately, when routine pneumococcal conjugate vaccination of infants and young children is accompanied by supportive education and active disease surveillance as well as judicious use of antibiotics, a favourable impact on the incidence of pneumococcal disease in and beyond the vaccinated population should be observed.
Top of page
References
1. Van Beneden CA, Whitney CG, Levine OS. Preventing pneumococcal disease among infants and young children. MMWR Morb Mortal Wkly Rep 2000;49:1-38.
2. Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J 2000;19:187-95.
3. McIntyre PB, Gilmour RE, Gilbert GL, Kakakios AM, Mellis CM. Epidemiology of invasive pneumococcal disease in urban New South Wales, 1997-1999. Med J Aust 2000;173 Suppl:S22-S26.
4. Torzillo PJ, Hanna JN, Morey F, Gratten M, Dixon J, Erlich J, et al. Invasive pneumococcal disease in Central Australia. Med J Aust 1995;162:182-186.
5. Fagan RL, Hanna JN, Messer RD, Brookes DL, Murphy DM. The epidemiology of invasive pneumococcal disease in children in Far North Queensland. J Paediatr Child Health 2001;37:571-575.
6. Hogg GG, Strachan JE, Lester RA. Invasive pneumococcal disease in the population of Victoria. Med J Aust 2000;173 Suppl:S32-S35.
7. Liddle JL, McIntyre PB, Davis CW. Incidence of invasive pneumococcal disease in Sydney children, 1991-96. J Paediatr Child Health 1999;35:67-70.
8. Dowell SF, Schwartz B. Resistant pneumococci: protecting patients through judicious use of antibiotics. Am Fam Physician 1997;55:1647-1648.
9. Collignon PJ, Turnidge JD. Antibiotic resistance in Streptococcus pneumoniae. Med J Aust 2000;173 Suppl:S58-S64.
10. Hausdorff WP, Bryant J, Paradiso PR, Siber GR. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin Infect Dis 2000;30:100-121.
11. Gratten M, Carlisle J, Hanna J, Bapty G, George N, Nuttall N, et al. Seroepidemiology of invasive pneumococcal disease in Queensland, 1990 to 1997. Commun Dis Intell 1998;22:265-269.
12. Butler JC, Bulkow LR, Parks DJ, et al. Epidemiology of pneumococcal bacteremia and meningitis during the first 5 years of life in Alaska: implications for conjugate pneumococcal vaccine use [abstract]. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy 2000;672.
13. Syrjänen R, Kilpi T, Herva E, et al. Pneumococcal carriage during health and respiratory infection [abstract]. International Symposium on Pneumococci and Pneumococcal Disease Abstract Book 1998;29.
14. Appelbaum PC, Gladkova C, Hryniewicz W, Kojouharov B, Kotulova D, Mihalcu F, et al. Carriage of antibiotic-resistant Streptococcus pneumoniae by children in eastern and central Europe-a multi-center study with use of standardized methods. Clin Infect Dis 1996;23:712-717.
Top of page
15. Garcia-de-Lomas J, Gimeno C, Millas E, Bermejo M, Lazaro MA, Navarro D, et al. Antimicrobial susceptibility of Streptococcus pneumoniae isolated from pediatric carriers in Spain. Eur J Clin Microbiol Infect Dis 1997;16:11-13.
16. Shackley F, Heath PT, Diggle L, et al. Carriage of Streptococcus pneumoniae (Spn) in Oxford children [abstract]. International Symposium on Pneumococci and Pneumococcal Diseases 1998;36.
17. Skull SA, Shelby-James T, Morris PS, Perez GO, Yonovitz A, Krause V, et al. Streptococcus pneumoniae antibiotic resistance in Northern Territory children in day care. J Paediatr Child Health 1999;35:466.
18. Nasrin D, Collignon PJ, Roberts L, Wilson EJ, Pilotto LS, Douglas RM. Effect of beta-lactam antibiotic use in children on pneumococcal resistance to penicillin: prospective cohort study. BMJ 2002;324:28-30.
19. Butler JC, Dowell SF, Breiman RF. Epidemiology of emerging pneumococcal drug resistance: implications for treatment and prevention. Vaccine 1998;16:1693-1697.
20. Gosbell IB, Neville SA. Antimicrobial resistance in Streptococcus pneumoniae: a decade of results from south-western Sydney. Commun Dis Intell 2000;24:340-343.
21. National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing; 11th informational supplement. National Committee for Clinical Laboratory Standards, Wayne, Pennsylvania, 2001;21:1-123.
22. American Academy of Paediatrics Committee on Infectious Diseases. Therapy for children with invasive pneumococcal infections. Paediatrics 1997;99:289-299.
23. Fenoll A, Martin BC, Munoz R, Vicioso D, Casal J. Serotype distribution and antimicrobial resistance of Streptococcus pneumoniae isolates causing systemic infections in Spain, 1979-1989. Rev Infect Dis 1991;13:56-60.
24. Fenoll A, Jado I, Vicioso D, Berron S, Yuste JE, Casal J, et al. Streptococcus pneumoniae in children in Spain: 1990-1999. Acta Paediatr 2000;89 Suppl:44-50.
25. Doern GV, Pfaller MA, Kugler K, Freeman J, Jones RN. Prevalence of antimicrobial resistance among respiratory tract isolates of Streptococcus pneumoniae in North America: 1997 results from the SENTRY antimicrobial surveillance program. Clin Infect Dis 1998;27:764-770.
26. Turnidge JD, Bell JM, Collignon PJ. Rapidly emerging antimicrobial resistance in Streptococcus pneumoniae in Australia. Med J Aust 1999;170:152-155.
Top of page
27. Jacobs MR, Bajaksouzian S, Zilles A, Lin G, Pankuch GA, Appelbaum PC, et al. Susceptibilities of Streptococcus pneumoniae and Haemophilus influenzae to 10 oral antimicrobial agents based on pharmacodynamic parameters: 1997 U.S. Surveillance Study. Antimicrob Agents Chemother 1999;43:1901-1908.
28. Gratten M, Nimmo G, Carlisle J, Schooneveldt J, Seneviratne E, Kelly R, et al. Emergence of further serotypes of multiple drug-resistant Streptococcus pneumoniae in Queensland. Commun Dis Intell 1997;21:133-136.
29. Arnold KE, Leggiadro RJ, Breiman RF, Lipman HB, Schwartz B, Appleton MA, et al. Risk factors for carriage of drug-resistant Streptococcus pneumoniae among children in Memphis, Tennessee. J Pediatr 1996;128:757-764.
30. Levine OS, Farley M, Harrison LH, Lefkowitz L, McGeer A, Schwartz B, et al. Risk factors for invasive pneumococcal disease in children: a population-based case-control study in North America. Pediatrics 1999;103:E28.
31. Givon-Lavi N, Dagan R, Fraser D, Yagupsky P, Porat N. Marked differences in pneumococcal carriage and resistance patterns between day care centers located within a small area. Clin Infect Dis 1999;29:1274-1280.
32. Black S, Shinefield H. Issues and challenges: pneumococcal vaccination in paediatrics. Paediatr Ann 1997;26:355-360.
33. Black SB, Shinefield HR, Hansen J, Elvin L, Laufer D, Malinoski F, et al. Postlicensure evaluation of the effectiveness of seven valent pneumococcal conjugate vaccine. Pediatr Infect Dis J 2001;20:1105-1107.
34. Eskola J, Kilpi T, Palmu A, Jokinen J, Haapakoski J, Herva E, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med 2001;344:403-409.
35. Dagan R, Givon-Lavi N, Shkolnik L, Yagupsky P, Fraser D. Acute otitis media caused by antibiotic-resistant Streptococcus pneumoniae in southern Israel: implication for immunizing with conjugate vaccines. J Infect Dis 2000;181:1322-1329.
36. Shinefield H. Pneumococcal conjugate vaccines and ongoing lessons. Int J Clin Pract 2001;118 Suppl:23-25.
37. Dagan R, Sikuler-Cohen M, Zamir O, Janco J, Givon-Lavi N, Fraser D. Effect of a conjugate pneumococcal vaccine on the occurrence of respiratory infections and antibiotic use in day-care center attendees. Pediatr Infect Dis J 2001;20:951-958.
38. Information for Immunisation Providers National Childhood
Pneumococcal Vaccination Program. Available from: http://www.health.gov.au/pubhlth/immunise/publications.htm.
Accessed: May 2002.
39. Mbelle N, Huebner RE, Wasas AD, Kimura A, Chang I, Klugman KP. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J Infect Dis 1999;180:1171-1176.
40. O'Brien KL, Bronsdon MA, Carlone GM, et al. Effect of a 7-valent pneumococcal conjugate vaccine on nasopharyngeal (NP) carriage among Navajo and White Mountain Apache (N/WMA) infants [abstract]. 19th Annual Meeting of the European Society for Paediatric Infectious Diseases 2001;22.
41. Dagan R, Givon S, Yagupsky P, et al. Effect of a 9-valent pneumococcal CRM197 vaccine (PncCRM9) on nasopharyngeal (NP) carriage of vaccine type and non-vaccine type S. pneumoniae (Pnc) strains among day care center (DCC) attendees (abstract). Program and Abstracts of the 38th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy 1998;299.
42. Obaro SK, Adegbola RA, Banya WAS, Greenwood BM. Carriage of pneumococci after pneumococcal vaccination. Lancet 1996;348:271-272.
Top of page
Author affiliations
Correspondence: Dr Ron Dagan, Pediatric Infectious Disease Unit, Soroka University Medical Center, PO Box 151, Beer-Sheva 84101, Israel. Telephone: +011 972 8 6400547. Facsimile: +011 972 8 6232334. Email: rdagan@bgumail.bgu.ac.il
This article was published in Communicable Diseases Intelligence Volume 27 Suppl, May 2003.
